Research
Following are some examples of our recent research projects
1. Development of electrolytes for energy storage devices
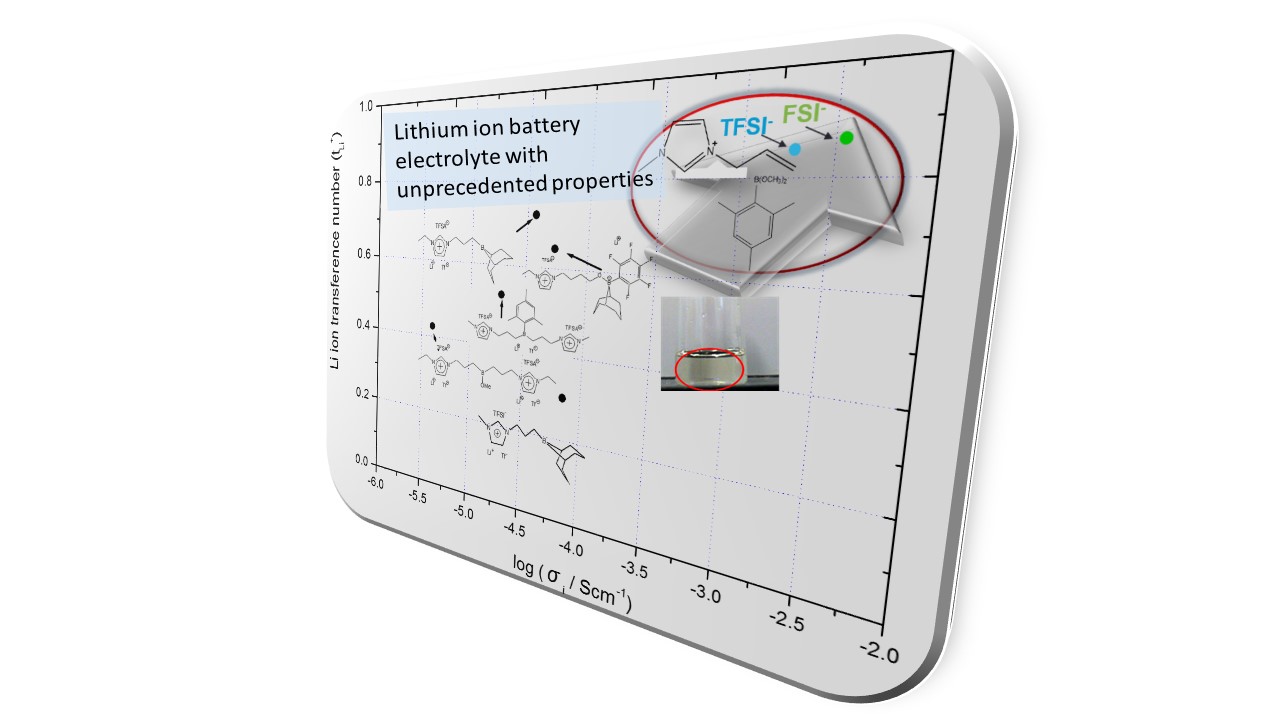
This research group is working on development of various electrolytes for application to next generation type energy storage devices. For instance, miscible binary electrolyte composed of ionic liquid/organoboron compound shows extremely high lithium transference number in spite of liquid nature of electrolytes. Incorporation of boron species was found to be effective for widening electrochemical window as well.
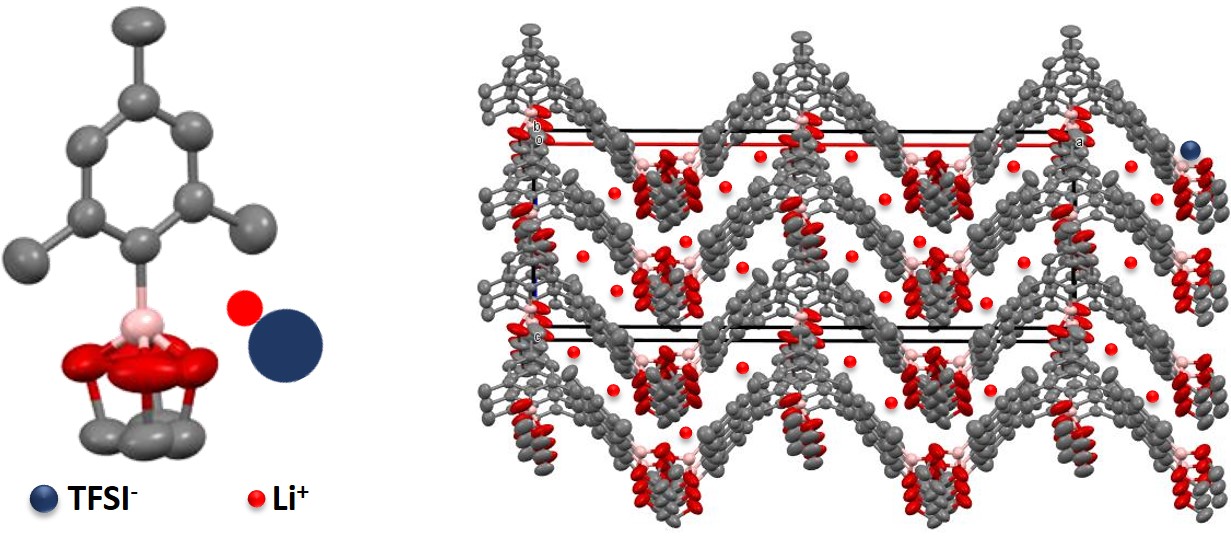
We also designed a new class of low molecular weight organoboron cyclic polymer electrolyte for the use in Li-ion and Na-ion secondary batteries as solid state electrolyte. In this system, interaction between crystalline boron compound and anion results in some structural order of resulting ion conductive path. At the same time, nano-channel like structure of crystalline boron compound is providing an ion conductive path for efficient ionic transportation. Particularly in the case of Na-ion secondary battery, this electrolyte shows suitability for high-rate charging-discharging due to specific SEI formation behavior of organoboron compound.
Further, we also succeeded in preparation of polymer ion-gel electrolytes exhibiting both high ionic conductivity and lithium transference number at the same time. This organoboron ion-gel electrolyte was prepared via lithiation of bio-based polybenzimidazole using LiH followed by complex formation between benzimidazolate anion and organoboron additive.
2. Rational Molecular Design of Polymer Binder for Energy Storage Devices, Part I
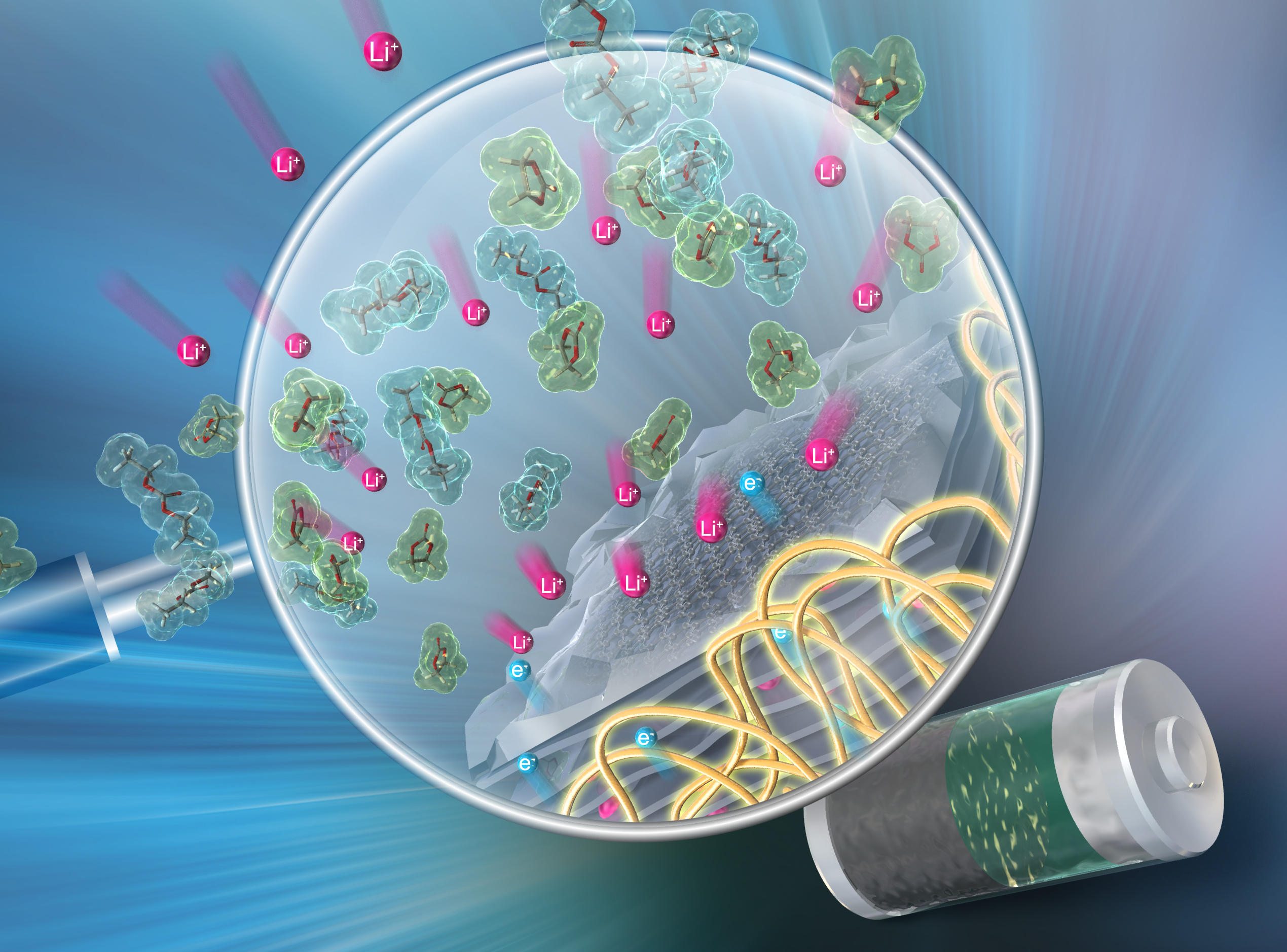
In energy storage systems, it has been widely recognized that not only electrolytes and active materials but also binder materials significantly affect the battery performance. Binder materials required various properties such as mechanical robustness, adhesion to current collector, miscibility with active materials, SEI forming property at electrode/electrolyte interface, solubility in slurry solution and so forth. Therefore, it is rational approach to strategically introduce each one of corresponding structure to the polymer binder material under task-specific design. Furthermore, in recent advanced design of polymer binder materials, it is also beneficial to design taking into account electronic structure of binder materials as it largely affects SEI formation behavior. In context of above-mentioned background, we have developed various high performing anodic binder materials such as polymerized ionic liquids and polymerized BIAN (bisiminoacenaphthene).
3. Rational Molecular Design of Polymer Binder for Energy Storage Devices, Part II
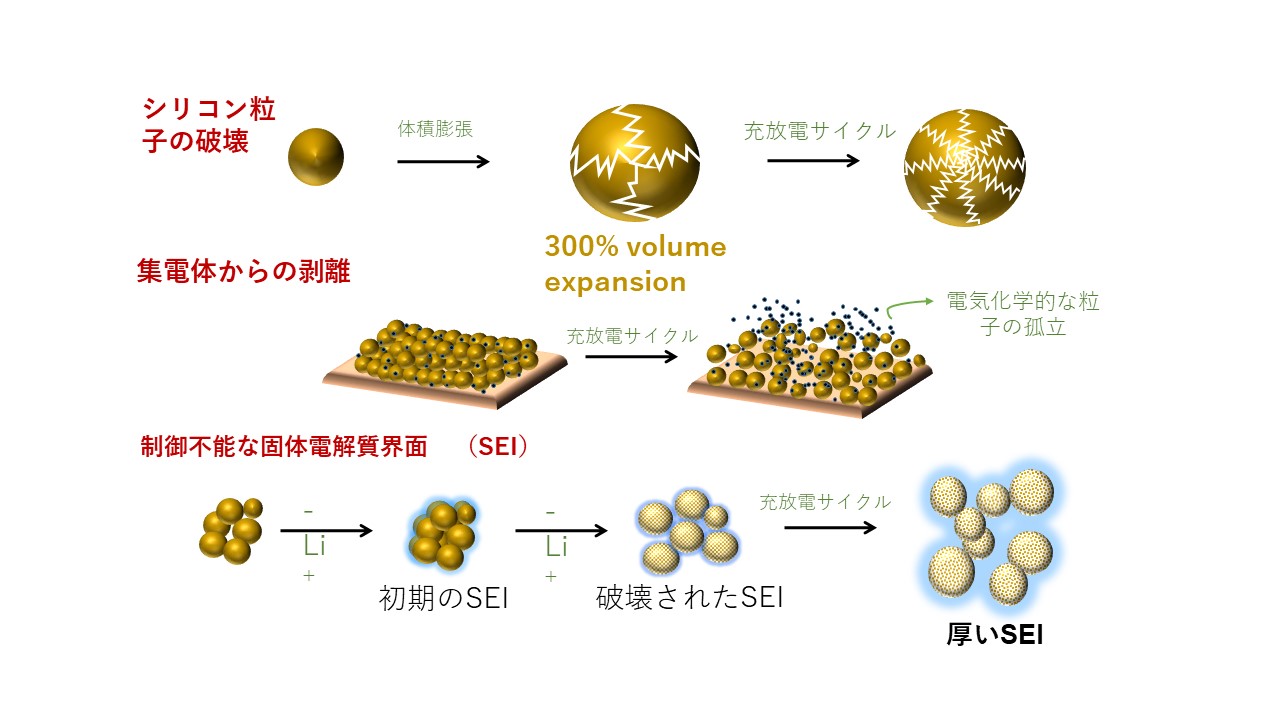
In recent years, as high capacity anodic active materials for Li ion secondary batteries, Si based materials are attracting intense interest due to elemental abundance, low cost and very high theoretical capacity. However, silicone particles undergo very large volumetric changes during charging and discharging, resulting in decomposition of particles and interface, exfoliation from current collector, crack formation, active decomposition of electrolyte at newly formed Si surface to form thick SEI layer. This leads to very high internal resistance and declined performance of battery cells. In order to highly stabilizing Si based anode, we are designing a variety of specific polymer coating materials and polymer binder materials (such as self-healing polymer binders) and successfully stabilized Si based anode to enable ultrahigh durability.
4. Development of Anodic Active Material Suitable for High-rate Charging
Nowadays, much attention has been focused on high-rate charging technology in Li-ion secondary batteries. We recently found that highly nitrogen-doped carbon material prepared by calcination of highly thermostable bio-based polybenzimidazole shows excellent capability for high-rate charging when applied as anodic active material in Li-ion battery. Heavy nitrogen doping enabled defect introduction and expansion of interlayer spacing, resulting in better diffusion of Li-ions in system and suitability for high-rate charging.
5. Development of Anodic Active Material of High Capacity and Durability
Very recently, we reported a new class of high capacity anodic active material which shows highly durable cycles even in the presence of conventional binder materials. β-SiC/N-doped carbon composite exhibited much lesser volumetric change during charge-discharge. Anodic half cell fabricated using β-SiC/N-doped as anode maintained ~1200 mAhg-1 of discharging capacity after 300 cycles.

6. Developmemt of Electrocatalysts
We are working on development of electrocatalysts for oxygen reduction reaction and oxygen evolution reaction which are essential for Li air batteries. For example, electrocatalyst composed of exfoliated acetylene black/Pt nanoparticles showed remarkably high electrochemical activity in comparison with commercial catalysts due to high amount of functional groups in exfoliated acetylene black, strong metal-substrate interaction, low interfacial resistance due to highly solvent absorbing nature of exfoliated acetylene black. We also reported green synthesis of electrocatalysts by photoreaction in aqueous solution. Recently we are also trying to control electronic structure of electrocatalysts by electropolymerizing thiophene derivatives on TiO2 nanotube before loading metal nanoparticles.
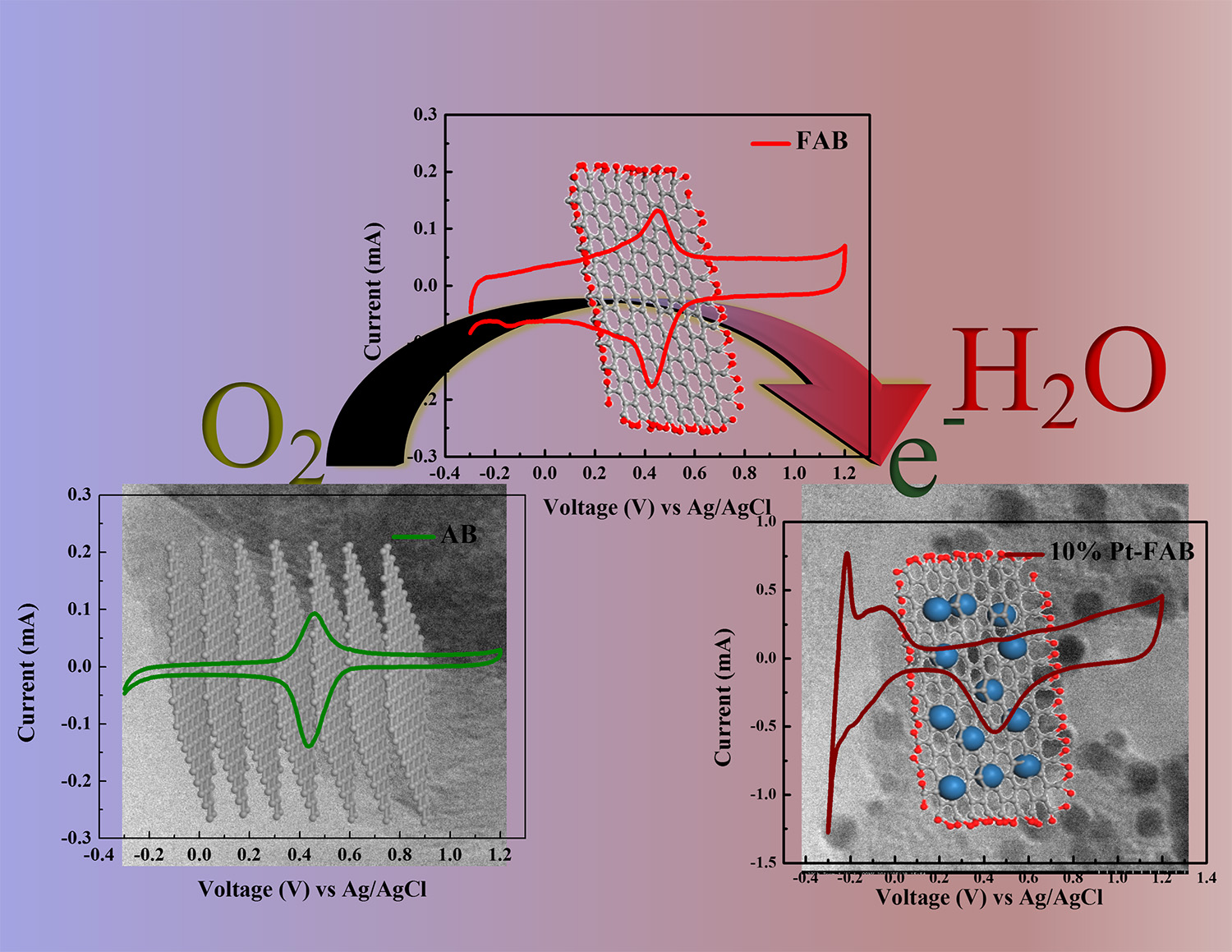
In addition to above-mentioned research projects, we are also working on supercapacitors, Li-sulfur batteries, additive to stabilize Li-ion battery cathode and so forth, from a variety of angles.

