研究業績
Papers | Books | Patents | Medias | Presentations
Papers List
2025
- [6]
- Nano-bismuth vanadate supported on fibrous silica reduced the intrinsic charge impedance for superior photoelectrochemical water-splitting performance
- N. M.Izzudin, A. A. Jalil, S. Rajendran, N. S. Hassan, M. H. Sawal, N. I. H. Hazril, Y. Nagao, K. Aoki , S. Zein
Nanoscale, accepted, (2025)
DOI: 10.1039/D4NR05153J
- [5]
- Molecular dynamics simulation of polymer electrolyte membrane for understanding structure and proton conductivity at various hydration levels using neural network potential
- A. Taborosi*, K. Aoki, N. Zettsu, M. Koyama, Y. Nagao*
Macromolecules, 58, 3720 - 3727 (2025)
DOI: 10.1021/acs.macromol.4c02607
- [4]
- Rhodium-Modified Glassy Carbon Electrode as a Promising Electrocatalyst for Oxygen Reduction Reaction in Phosphoric Acid Electrolytes
- S. M. N. Uddin, A. Y. Abir, M. I. Hossain, N. Akter, M. B. Islam, K. Aoki, Y. Nagao, S. S. Makedar, M. Rahaman, M. A. Hasnat
ChemNanoMat, accpeted, e202400468 (2025)
DOI: 10.1002/cnma.202400468
- [3]
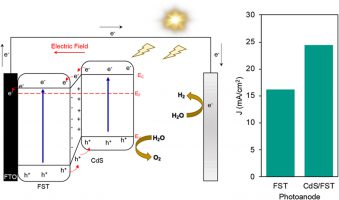
- n-n heterojunction CdS/FST photoanode for enhanced photoelectrochemical water splitting
- M. H. Sawal, A. A. Jalil, T. A. T. Abdullah, N. S. Hassan, M. B. Bahari, N. M. Izzudin, N. W. C. Jusoh, Y. Nagao, K. Aoki, M. N. Chong, S. Rajendran
Int. J. Hydrog. Energy, 104, 336 - 343 (2025)
DOI: 10.1016/j.ijhydene.2024.03.215
- [2]
- Deep eutectic solvent as simultaneous supporting electrolyte and structure-directing agent in electrosynthesis of ZnO for enhanced visible-light chlorophenol degradation
- A. A. A. Mutalib, N. F. Jaafar, N. W. C. Jusoh, Y. Nagao, K. Aoki, N. Matsumi, S. Nishimura
J. Mol. Liq., 422, 126956 (16 pages) (2025)
DOI: 10.1016/j.molliq.2025.126956

- [1]
- Isomorphously Substituted Cerium Induced Oxygen Vacancy and Medium Basicity in Ni/Fibrous Silica Catalyst for superior low-temperature CO2 Methanation
- M. A. Aziz, A. A. Jalil, N. S. Hassan, M. B. Bahari, T. A. T. Abdullah, N. W. C. Jusoh, Y. Nagao, K. Aoki, S. Nishimura, R. Saravanan
Appl. Catal. A Gen., 689, 120019 (11 pages) (2025)
DOI: 10.1016/j.apcata.2024.120019
2024
- [19]
- Reversible Photoswitching of Proton Conduction in Hetero-Smectic Lamellar Structures Formed by Side-Chain Liquid Crystalline Copolymer Thin Films
- Y. Ishizaki-Betchaku, K. Suetsugu, M. Hara, Y. Nagao, J. Matsui, T. Seki, S. Nagano
Polym. Int., accepted, (2024)
DOI: 10.1002/pi.6741
- [18]
- Synergistic photocatalysis: Metal oxide composites for enhanced wastewater treatment
- N. A. F. R. Madi, N. W. C. Jusoh, L. S. Tan, M. F. M. Nordin, Y. Nagao
Ingeniare, 32, 35 (12 pages) (2024)
- [17]
- Effects of Alkyl Side Chain Length on the Structural Organization and Proton Conductivity of Sulfonated Polyimide Thin Films (Selected as Cover)
- T. Honbo, Y. Ono, K. Suetsugu, M. Hara, A. Taborosi, K. Aoki, S. Nagano, M. Koyama, Y. Nagao*
ACS Appl. Polym. Mater., 6, 13217 - 13227 (2024)
DOI: 10.1021/acsapm.4c02490

- [16]
- Optimization of the Synergistic Effects in Polycrystalline Pt-Au Electrodes in Developing an Effective Arsenic Sensor via Oxidation Reactions
- M. I. Hossain, S. R. Saha, K. Aoki, Md. M. Alam, N. R. Singha, M. Rahaman, Ali Aldalbahi, Y. Nagao, M. A. Hasnat
New J. Chem., 48, 18301 - 18313 (2024)
DOI: 10.1039/D4NJ03312D
- [15]
- Enhancing H+ Conduction through Glycolic Acid-Doped Alginate-PVA Biopolymer Electrolytes
- N. M. Ghazali, K. Aoki, Y. Nagao, A. S. Samsudin
Int. J. Hydrog. Energy, 89, 177 - 189 (2024)
DOI: 10.1016/j.ijhydene.2024.09.244
- [14]
- Ti3C2Tx/MoO3 Heterostructure As An Ultrasensitive And Selective Sensing Material For A Room-Temperature Nitrogen Dioxide Sensor
- L. C. T. Cao , P.-S. Chen , Y.-H. Lin , Y. Nagao, S. Boonruang, C.-A. Jong, S.-H. Hsu
Appl. Surf. Sci., 676, 161025 (10 pages) (2024)
DOI: 10.1016/j.apsusc.2024.161025

- [13]
- Influence of Different Dissolved Gases on Electrocatalytic Nitrate Sensing Performance at Cu-modified Au Electrode
- M. B. Islam, M. I. Hossain, N. Hosen, M. Rahaman, N. R. Singha, K. Aoki, Y. Nagao, M. A. Hasnat
ChemistrySelect, 9, e202402986 (12 pages) (2024)
DOI: 10.1002/slct.202402986
- [12]
- Electrokinetics of nitrite to ammonia conversion in the neutral medium over a platinum surface
- Md. F. Islam, H. S. Ifti, M. Rahman, K. Aoki, Y. Nagao, A.Aldalbahi, J. Uddin , M. A. Hasnat
Chem. Asian J., e202400362 (9 pages) (2024)
DOI: 10.1002/asia.202400362

- [11]
- Enhancing Electrochemical Performance of Alginate–PVA Solid Blend Electrolytes via H+ Ion Doping for Supercapacitor Applications
- N. M. Ghazali, N. F. Mazuki, M. H. Sulaiman, K. Aoki, Y. Nagao, A. S. Samsudin
Solid State Ionics, 414, 116650 (15 pages) (2024)
DOI: 10.1016/j.ssi.2024.116650
- [10]
- The ionic conductivity and electrochemical performance of Alginate-PVA based polymer electrolyte with Li+ charge carriers for supercapacitor
- A. S. Samsudin, N. M. Ghazali, N. F. Mazuki , K. Aoki, Y. Nagao
J. Electroanal. Chem., 967, 118463 (18pages) (2024)
DOI: 10.1016/j.jelechem.2024.118463

- [9]
- Cross-linkable Sulfonated Polyimide Thin Film With High Proton Conductivity [conference proceeding]
- Y. Yao, M. Hara, S. Nagano, K. Aoki, Y. Nagao*
Proc. STEPI-12, 12, 49 - 59 (2024)
Repository: http://hdl.handle.net/10119/19046

- [8]
- Utilizing metal oxide/fabric composites for photocatalytic degradation of wastewater [conference proceeding]
- N. A. F. R. Madi, N. W. C. Jusoh, Y. Nagao, L. S. Tan, M. F. M. Nordin
E3S Web Conf., 516, 03004 (5 pages) (2024)
DOI: 10.1051/e3sconf/202451603004
- [7]
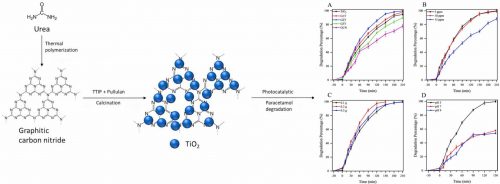
- Enhanced paracetamol photodegradation over synthesized TiO2/g-C3N4 nanocomposites: Effect of g-C3N4 loading on the properties and performance
- E. D. M. Isa, R. R. Ali, N. W. C. Jusoh, Y. Nagao, K. Aoki, S. Nishimura, Z. I. A. Tarmizi, S. H. M. Taib
Colloids Surf. A: Physicochem. Eng. Asp., 134066 (11 pages) (2024)
DOI: 10.1016/j.colsurfa.2024.134066
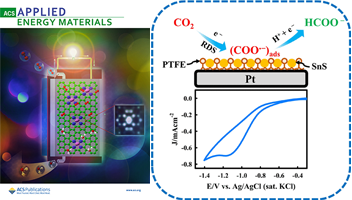
- [6]
- Electrochemical Reduction of CO2 by SnS⎸PTFE⎸Pt Surface in an Aqueous Imidazole Medium: Catalysis and Kinetics
- Md. T. Islam, M. I. Hossain, K. Aoki, Y. Nagao, Md. M, Hasan, M. Rahaman, A. Aldalbahi, M. A. Hasnat
ACS Appl. Energy Mater., 7, 3125 - 3136 (2024)
DOI: 10.1021/acsaem.3c03142
- [4]
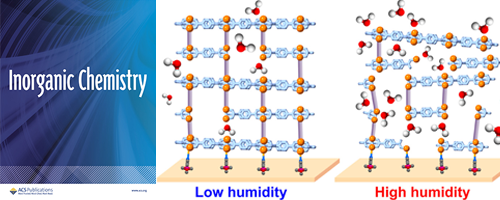
- Influence of Humidity on Layer-by-Layer Growth and Structure in Coordination Networks
- K. Aoki, T. Matsuzawa, K. Suetsugu, M. Hara, S. Nagano, Y. Nagao*
Inorg. Chem., 63, 6674 - 6682 (2024)
DOI: 10.1021/acs.inorgchem.3c04526
In this study, we fabricated MOF thin films composed of Zn2+, tetrakis-(4-carboxyphenyl)-porphyrin (TCPP), and 4,4′-bipyridyl (bpy) at 10 and 40% relative humidity (RH) conditions. Then, we investigated the humidity effects on chemical compositions of TCPP and bpy, periodic structure, orientation, and surface morphology. At high RH, coordination replacement of water with the organic linkers becomes more competitive than that at low RH, resulting in a different TCPP/bpy composition ratio between the two RH conditions.
- [3]
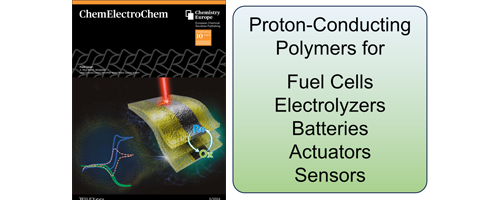
- Proton-Conducting Polymers: Key to Next-Generation Fuel Cells, Electrolyzers, Batteries, Actuators, and Sensors (Review)
- Y. Nagao*
ChemElectroChem, 11, e202300846 (27 pages) (2024)
DOI: 10.1002/celc.202300846
The author summarized recent diverse applications and advancements for proton-conducting polymers since 2018, emphasizing their importance in various technological areas. These polymers are integral to fuel cells, water electrolysis, energy storage systems, actuators, and sensors, offering high proton conductivity, chemical stability, and adaptability.
- [2]
- Enhancement on H+ carriers in Conduction Properties with the addition of 1-Butyl-3-Methylimidazolium Chloride based Alginate Polymer Electrolytes
- A. F. Fuzlin, N. F. Mazuki, Md. M. Hasan, Y. Nagao, A. S. Samsudin
Int. J. Hydrog. Energy, 60, 201 - 211 (2024)
DOI: 10.1016/j.ijhydene.2024.02.154
- [1]
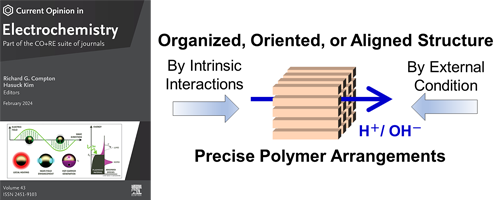
- Advancing Sustainable Energy: Structurally Organized Proton and Hydroxide Ion-Conductive Polymers (Review)
- Y. Nagao*
Curr. Opin. Electrochem., 44, 101464 (9 pages) (2024)
DOI: 10.1016/j.coelec.2024.101464
This concise review paper specifically described advancements in proton and hydroxide ion-conductive polymers, emphasizing their structural organization and molecular orientation by various intrinsic molecular interactions and external environmental conditions.
2023
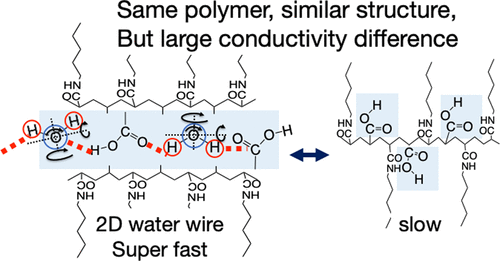
- [11]
- Mechanism of High Proton Mobility in the Two-Dimensional Nanospace at the Interlayer of a Multilayer Polymer Nanosheet Film
- M. Inoue, R. Sakashita, S. Kagaya, M. Gemmei-Ide, Y. Yao, A. Suwansoontorn, S. Nagano, S. Yamamoto, M. Mitsuishi, Y. Nagao, J. Matsui
J. Phys. Chem. C, 127, 24046 - 24055 (2023)
DOI: 10.1021/acs.jpcc.3c06848
- [10]
- IrOx–Pt electrode for the electro-oxidation of ethanol in alkaline-type direct ethanol fuel cells: an excellent CO-tolerant catalyst
- Md. F. Islam, J, Ahmed, M. Faisal, J. S. Algethami, K. Aoki, Y. Nagao, F. A. Harraz, M. A. Hasnat
New J. Chem., 47, 18933 - 18944 (2023)
DOI: 10.1039/D3NJ03306F
- [9]
- Photocatalytic degradation of chlorazol yellow dye under sunlight irradiation using Ce, Bi, and N co-doped TiO2 photocatalyst in neutral medium
- Z. M. Moushumy, M. J. Hassan, M. Ahsan, M. M. Hasan, M. N. Uddin, Y. Nagao, M. A. Hasnat
Environ. Sci. Pollut. Res., 30, 35153 - 35169 (2023)
DOI: 10.1007/s11356-022-24220-0
- [8]
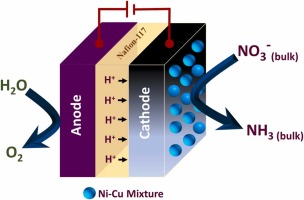
- Development of CuNi immobilized platinum surface to minimize nitrite evolution during electrocatalytic nitrate reduction in neutral medium
- Md. N. Islam, M. Ahsan, K. Aoki, Y. Nagao, A. E. Alsafrani, H. M. Marwani, A. Almahri, M. M. Rahman, M. A. Hasnat
J. Environ. Chem. Eng., 11, 111149 (11 pages) (2023)
DOI: 10.1016/j.jece.2023.111149
- [7]
- Kinetics of electrocatalytic oxidation of Gallic acid by activated glassy carbon electrode in acidic medium
- M. Siddika, J. Ahmed, K. Aoki, M. Faisal, J. S. Algethami, F. A. Harraz, Y. Nagao, M. A. Hasnat
ChemistrySelect, 8, e20230207 (11 pages) (2023)
DOI: 10.1002/slct.202302074
- [6]
- Efficient Electrocatalytic Hydrogen Evolution Reaction on CuO Immobilized on a Stainless-Steel Electrode Prepared by the SILAR Method
- Md. N. Islam, J. Ahmed, M. Faisal, J. Algethami, K. Aoki, Y. Nagao, F. A. Harraz, M. A. Hasnat
ChemistrySelect, 8, e202301077 (10 pages) (2023)
DOI: 10.1002/slct.202301077
- [5]
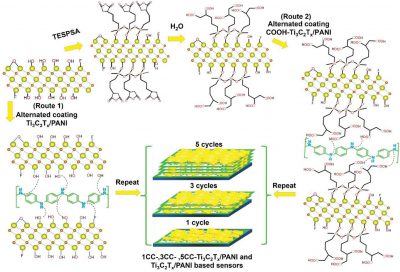
- Facile Fabrication Of Oxygen-enriched MXene Based Sensor And Their Ammonia Gas-sensing Enhancement
- L. C. T. Cao, M-H. Zhou, P. Opaprakasit, P. Sreearunothai, Y. Nagao, S. Boonruang, H. Fallah, S-F. Tseng, S. H. Hsu
Adv. Mater. Interfaces, 10, 2300166 (13 pages) (2023)
DOI: 10.1002/admi.202300166
- [4]
- Novel polymer electrolyte based polyurethane acrylate for low-cost perovskite solar cells applications
- I. M. Noor, Z. E. Rojudi, M. I. M. Ghazali, N. Tamchek, Y. Nagao
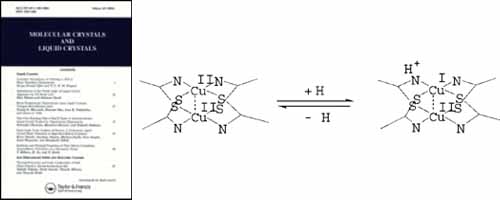
Mol. Cryst. Liq. Cryst., 761, 7 - 21 (2023)
DOI: 10.1080/15421406.2023.2173770
- [3]
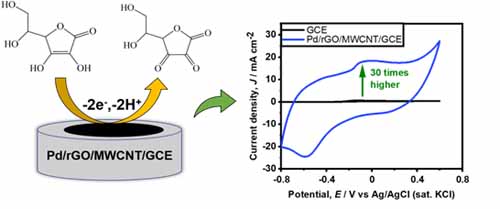
- Palladium nanoparticles combined with reduced graphene oxide and multiwall carbon nanotubes for alkaline ascorbic acid oxidation
- Md. M. Hasan, Z. Li, Y. Nagao*
Jpn. J. Appl. Phys., 62, 027003 (8 pages) (2023)
DOI: 10.35848/1347-4065/acb897
- [2]
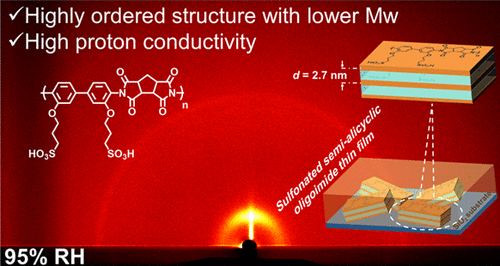
- Lyotropic Liquid Crystalline Property and Organized Structure in High Proton-Conductive Sulfonated Semi-Alicyclic Oligoimide Thin Films
- Y. Yao, H. Watanabe, M. Hara, S. Nagano, Y. Nagao*
ACS Omega, 8, 7470 - 7478 (2023)
DOI: 10.1021/acsomega.2c06398
- [1]
- Designed Fabrication of Active Tumor Targeting Covalent Organic Framework Nanotherapeutics via a Simple Post-Synthetic Strategy
- Y. Yu, G. Zhang, Z. Li, J. Wang, Y. Liu, R. Bhardwaj, R. Wadhwa, Y. Nagao, M. Shichiri, R. Gao
Nano Res., 16, 7085 - 7094 (2023)
DOI: 10.1007/s12274-022-5265-7
2022
- [10]
- Studies on H+ ions conducting bio-polymer blend electrolyte based on alginate-PVA doped with NH4NO3
- N. M. Ghazali, A. F. Fuzlin, M. A. Saadiah, Md. M. Hasan, Y. Nagao, A. S. Samsudin
J. Non-Cryst. Solids, 598, 121939 (15 pages) (2022)
DOI: 10.1016/j.jnoncrysol.2022.121939
- [9]
- Potential use of Gold-Silver Core-Shell Nanoparticles derived from Garcinia mangostana Peel for Anticancer Protocatechuic Acid Delivery
- K. X. Lee, K. Shameli, Y. Nagao, Y. P. Yew, S.-Y. Teow, H. Moeini
Front. Mol. Biosci., 9, 997471 (15 pages) (2022)
DOI: 10.3389/fmolb.2022.997471
- [8]
- Attribution of H+ Transportation of Nafion-Film on Different Substrates Using Planar Inter-Digitated Electrodes
- R. Bhardwaj, K. Karan, Y. Nagao*
ECS Trans., 109, 303 - 316 (2022)
DOI: 10.1149/10909.0303ecst
- [7]
- Hydration and OH-/Br- Conduction Properties of Fluorene-Thiophene-Based Anion Exchange Thin Films Tethered with Different Cations
- F. Wang, S. Nagano, M. Hara, Y. Nagao*
ACS Appl. Polym. Mater., 4, 5965 - 5974 (2022)
DOI: 10.1021/acsapm.2c00811
- [6]
- Studies on the ions transportation behavior of alginate doped with H+ carrier-based polymer electrolytes
- A. F. Fuzlin, N. F. Mazuki, N. M. Khan, M. A. Saadiah, Md. M. Hasan, Y. Nagao, A. S. Samsudin
Mater. Chem. Phys., 287, 126207 (12 pages) (2022)
DOI: 10.1016/j.matchemphys.2022.126207
- [5]
- Study on ionic conduction of alginate bio-based polymer electrolytes by incorporating ionic liquid
- A. F. Fuzlin, I. I. Misnon, Y. Nagao, A. S. Samsudin
Mater. Today Proc., 51, 1455 - 1459 (2022)
DOI: 10.1016/j.matpr.2021.11.654
- [4]
- Facile fabrication of GCE/Nafion/Ni composite, a robust platform to detect hydrogen peroxide in the basic medium via oxidation reaction
- Md. F. Islam, Md. T. Islam, Md. M. Hasan, M. M. Rahman, Y. Nagao, M. A. Hasnat
Talanta, 240, 123202 (11 pages) (2022)
DOI: 10.1016/j.talanta.2021.123202
- [3]
- Involvement of ethylene carbonate on the enhancement H+ carriers in structural and ionic conduction performance on alginate bio-based polymer electrolytes
- A. F. Fuzlin, M. A. Saadiah, Md. M. Hasan, Y. Nagao, I. I. Misnon, A. S. Samsudin
Int. J. Hydrog. Energy, 47, 7846 - 7860 (2022)
DOI: 10.1016/j.ijhydene.2021.12.124
- [2]
- Accumulation of sulfonic acid groups anchored in covalent organic frameworks as an intrinsic proton-conducting electrolyte (Selected as Cover, 10 most downloaded papers)
- L. Zhai, Y. Yao, B. Ma, Md M. Hasan, Y. Han, L. Mi, Y. Nagao*, Z. Li*
Macromol. Rapid Commun., 43, 2100590 (7 pages) (2022)
DOI: 10.1002/marc.202100590
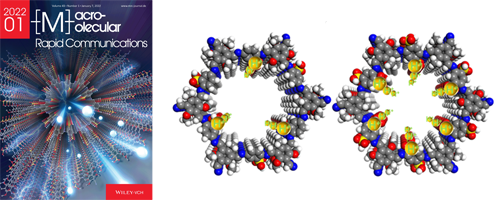
- [1]
- Correlation Studies Between Structural and Ionic Transport Properties of Lithium-ion Hybrid Gel Polymer Electrolytes based PMMA-PLA
- N. F. Mazuki, M. Z. Kufian, Y. Nagao, A. S. Samsudin
J. Polym. Environ., 30, 1864 - 1879 (2022)
DOI: 10.1007/s10924-021-02317-w
2021
- [13]
- Halide Replacement Effect on Proton Conductivity and Vapochromic Luminescence of Pt(II) Complexes
- A. Kobayashi, S. Imada, Y. Yao, Y. Nagao, Y. Kubota, M. Yoshida, M. Kato
Bull. Chem. Soc,. Jpn., 94, 2466 - 2473 (2021)
DOI: 10.1246/bcsj.20210279
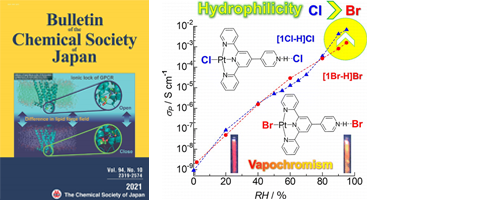
- [12]
- Efficient sensing of hydrogen peroxide via electrocatalytic oxidation reactions using polycrystalline Au electrode modified with controlled thiol group immobilization
- A. Dutta, Md. M. Hasan, Md. R. Miah, Y. Nagao, M. A. Hasnat
Electrochim. Acta, 395, 139217 (12 pages) (2021)
DOI: 10.1016/j.electacta.2021.139217
- [11]
- Intrinsic proton conduction in 2D sulfonated covalent organic framework through the post-synthetic strategy (selected as HOT articles)
- Y. Zhang, C. Li, Z. Liu, Y. Yao, Md. M. Hasan, Q. Liu, J. Wang, Z. Li*, H. Li*, Y. Nagao*
CrystEngComm, 23, 6234 - 6238 (2021)
DOI: 10.1039/D1CE00957E
- [10]
- Electrocatalytic oxidation of ammonia in the neutral medium using Cu2O.CuO film immobilized on glassy carbon surface
- Md. F. Shabik, Md. M. Hasan, K. A Alamry, M. M. Rahman, Y. Nagao, M. A. Hasnat
J. Electroanal. Chem., 897, 115592 (12 pages) (2021)
DOI: 10.1016/j.jelechem.2021.115592
- [9]
- Editing Light Emission with Stable Crystalline Covalent Organic Frameworks via Wall Surface Perturbation (Selected as Very Important Paper)
- Z. Li, K. Geng, T. He, K. T. Tan, N. Huang, Q. Jiang, Y. Nagao, D. Jiang
Angew. Chem. Int. Ed., 60, 19419 - 19427 (2021)
DOI: 10.1002/anie.202107179
A strategy to tune the emission properties of COFs by introducing atoms or small molecular groups as surface perturbations.
- [8]
- Ionic Conductivity Study of Ethylene Carbonate as A Plasticizer in Alginate Bio-Based Polymer Electrolytes
- A. F. Fuzlin, Y. Nagao, A. S. Samsudin
Macromol. Symp., 397, 2000236 (4 pages) (2021)
DOI: 10.1002/masy.202000236
- [7]
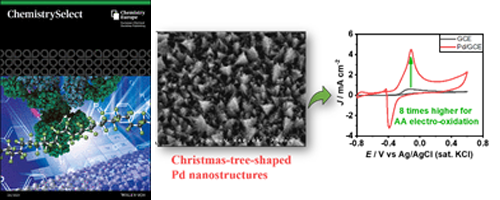
- Christmas-tree-shaped Palladium Nanostructures Decorated on Glassy Carbon Electrode for Ascorbic Acid Oxidation in Alkaline Condition (Selected as one of most discussed articles)
- Md. M. Hasan, Y. Nagao*
ChemistrySelect, 6, 5885 - 5892 (2021)
DOI: 10.1002/slct.202100974
The unique Christmas-tree-shaped Pd nanostructures were generated through a simple electrodeposition technique on the glassy carbon electrode (GCE). The modified electrode showed excellent catalytic activity than the unmodified GCE for ascorbic acid electro-oxidation in alkaline condition. This high efficiency is derived from the many hierarchical edges of nanostructures.
- [6]
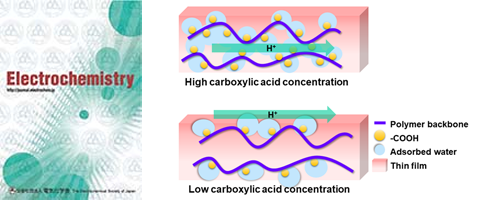
- Interfacial and internal proton conduction of weak-acid functionalized styrene-based copolymer with various carboxylic acid concentrations (Selected as Most Downloaded Papers)
- A. Suwansoontorn, K. Yamaomoto, S. Nagano, J. Matsui, Y. Nagao*
Electrochemistry, 89, 401 - 408 (2021)
DOI: 10.5796/electrochemistry.21-00042
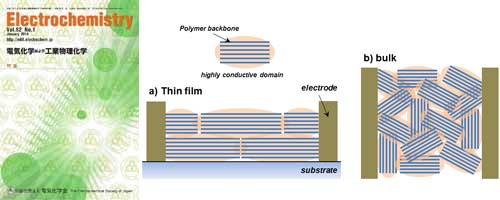
This study investigated the influence of carboxylic acid concentration on both interfacial and internal proton transport. Remarkably, polymer thin films with high carboxylic acid concentration provide internal proton conduction. In contrast, interfacial proton conduction was found in low carboxylic acid concentration polymers. This study provides insight into interfacial proton transport behavior according to the weak acid concentration, which might explain proton transport in biological systems.
- [5]
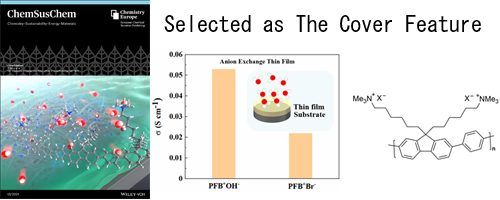
- OH- Conductive Properties and Water Uptake of Anion Exchange Thin Films (Selected as The Cover Feature)
- F. Wang, D. Wang, Y. Nagao*
ChemSusChem, 14, 2694 - 2697 (2021)
DOI: 10.1002/cssc.202100711
The Cover Feature shows the conduction of OH− ions in poly[(9,9-bis(6′-(N,N,N-trimethylammonium)-hexyl)-9H-fluorene)-alt-(1,4-benzene)] (PFB+) anion-exchange thin film. The OH− conductivity of PFB+ thin film is comparable to the reported OH− conductivity of PFB+ bulk membrane. Both reduced OH− conductivity and water uptake can be observed in thinner PFB+ film.
- [4]
- Enhancement on Protonation (H+) with Incorporation of Flexible Ethylene Carbonate in CMC–PVA–30 wt.% NH4NO3 Film
- M. A. Saadiah, Y. Nagao, A. S. Samsudin
Int. J. Hydrog. Energy, 46, 17231 - 17245 (2021)
DOI: 10.1016/j.ijhydene.2021.02.187

- [3]
- Preparation and structural characterization of shear aligned films for a high proton conductive alkyl sulfonated polyimide with lyotropic liquid crystallinity
- R. Goto, Y. Ono, M. Hara, T. Seki, Y. Nagao*, S. Nagano*
Mol. Cryst. Liq. Cryst., 727, 23 - 32 (2021)
DOI: 10.1080/15421406.2021.1946963
- [2]
- Oxygen Reduction Reaction in Layer-By-Layer Fabricated Cobalt Porphyrin-Based Nanostructures
- B. Lamlua, T. Ohyama, Y. Nagao*
Mater. Sci. Forum, 1025, 3 - 8 (2021)
DOI: 10.4028/www.scientific.net/MSF.1025.3
- [1]
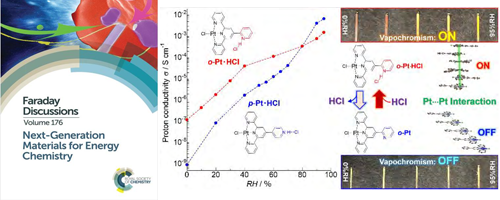
- Cooperative phenomenon of vapochromism and proton conduction of luminescent Pt(II) complexes for the visualisation of proton conductivity
- A. Kobayashi, S. Imada, D. Wang, Y. Nagao, M. Yoshida, M. Kato
Faraday Discuss., 225, 186 - 196 (2021)
DOI: 10.1039/D0FD00001A
2020
- [17]
- The influences of PLA into PMMA on crystallinity and thermal properties enhancement-based hybrid polymer in gel properties
- N. F. Mazuki, Y. Nagao, M. Z. Kufian, A. S. Samsudin
Mater. Today Proc., 49, 3105 - 3111 (2020)
DOI: 10.1016/j.matpr.2020.11.037
- [16]
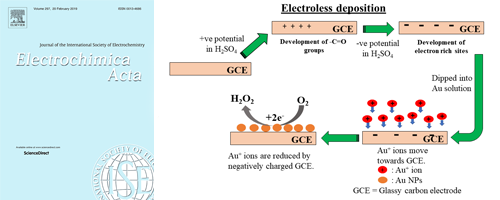
- Electroless deposition of gold nanoparticles on a glassy carbon surface to attain methylene blue degradation via oxygen reduction reactions
- Md. T. Islam, Md. M. Hasan, Md. F. Shabik, Md. F. Islam, Y. Nagao, M. A. Hasnat*
Electrochim. Acta, 360, 136966 (1-12) (2020)
DOI: 10.1016/j.electacta.2020.136966
- [15]
- Sulfonated Triazine-Based Porous Organic Polymer for Excellent Proton Conductivity
- Z. Li*, Z. Liu, H. Li, Md. M. Hasan, A. Suwansoontorn, G. Du, D. Wang, Y. Zhang, Y. Nagao*
ACS Appl. Polym. Mater., 2, 3267 - 3273 (2020)
DOI: 10.1021/acsapm.0c00425
- [14]
- A simple and cost-effective synthesis of ionic porous organic polymers with excellent porosity for high iodine capture
- Z. Li*, H. Li, D. Wang, A. Suwansoontorn, G. Du, Z. Liu, Md. M. Hasan, Y. Nagao*
Polymer, 204, 122796(1-7) (2020)
DOI: 10.1016/j.polymer.2020.122796
- [13]
- Alkaline stability of ether bond free fluorene based anion exchange polymer containing cycloaliphatic quaternary ammonium groups
- U. Salma, Y. Nagao*
Polym. Degrad. Stabil., 179, 109299(1-9) (2020)
DOI: 10.1016/j.polymdegradstab.2020.109299
- [12]
- Simple and universal synthesis of sulfonated porous organic polymers with high proton conductivity (Selected as hot paper)
- Z. Li*, Y. Yao, D. Wang, Md. M. Hasan, A. Suwansoontorn, H. Li*, G. Du, Z. Liu, Y. Nagao*
Mater. Chem. Front., 4, 2339 - 2345 (2020)
DOI: 10.1039/D0QM00276C
- [11]
- Ethylene Carbonate and Polyethylene Glycol as Efficient Plasticizers in CMC-PVA-NH4NO3-Based Polymer Electrolyte
- N. S. M. Ali, Y. Nagao, A. S. Samsudin
Makara J. Technol., 24, 13 - 17 (2020)
DOI: 10.7454/mst.v24i1.3833
- [10]
- Enhancing proton conductivity of sodium alginate doped with glycolic acid in bio-based polymer electrolytes system
- A. F. Fuzlin, M. A. Saadiah, Y. Yao, Y. Nagao, A. S. Samsudin
J. Polym. Res., 27, 207 (1-16) (2020)
DOI: 10.1007/s10965-020-02142-0
- [9]
- Proton (H+) Transport Properties of CMC-PVA Blended Polymer Solid Electrolyte Doped With NH4NO3
- M. A. Saadiah, Y. Nagao, A. S. Samsudin
Int. J. Hydrog. Energy, 45, 14880 - 14896 (2020)
DOI: 10.1016/j.ijhydene.2020.03.213

- [8]
- Electroless deposition of silver dendrite nanostructure onto glassy carbon electrode and its electrocatalytic activity for ascorbic acid oxidation
- Md. M. Hasan, R. H. Rakib, M. A. Hasnat*, Y. Nagao*
ACS Appl. Energy Mater., 3, 2907 - 2915 (2020)
DOI: 10.1021/acsaem.9b02513
Repository: http://hdl.handle.net/10119/17073
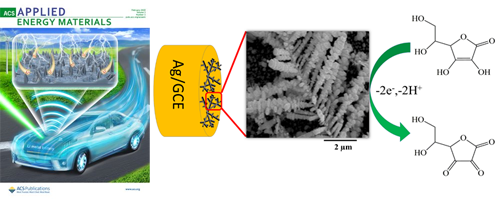
Well-defined silver dendrite nanostructures with primary and secondary branches on a glassy carbon electrode (GCE) surface are first demonstrated using a simple wet chemical electroless deposition method without any aid of a surfactant.
- [7]
- Progress on highly proton-conductive polymer thin films with organized structure and molecularly oriented structure (Review, Selected as Editor's choice)
- Y. Nagao*
Sci. Tech. Adv. Mater (STAM), 21, 79 - 91 (2020)
DOI: 10.1080/14686996.2020.1722740
Several current topics are introduced in this review, with particular attention to highly proton-conductive polymer thin films with organized structure and molecularly oriented structure.
- [6]
- Imidazolium functionalized fluorene based hydroxide ion conducting polymer for fuel cell applications
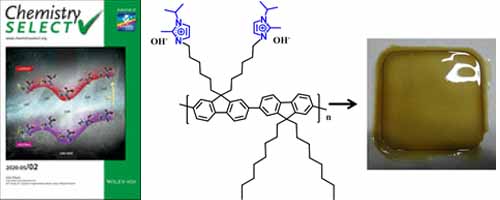
- U. Salma, D. Zhang, Y. Nagao*
ChemistrySelect, 5, 1255 - 1263 (2020)
DOI: 10.1002/slct.201903246
Repository: http://hdl.handle.net/10119/17076
- [5]
- Ion Transportation by Prussian Blue Nanoparticles Embedded in a Giant Liposome
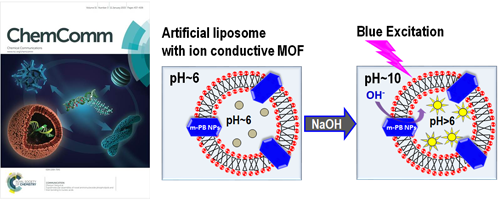
- S. M. N. Uddin, S. Laokroekkiat, M. A. Rahsed, S. Mizuno, K. Ono, M. Ishizaki, K. Kanaizuka, M. Kurihara, Y. Nagao*, T. Hamada*
Chem. Commun., 56, 1046 - 1049 (2020)
DOI: 10.1039/c9cc06153c
Repository: http://hdl.handle.net/10119/17074
- [4]
- Cross-Correlated Humidity Dependent Structural Evolution of Nafion Thin Film Confined on Platinum Substrate (selected as Soft Matter Most Popular 2020 collection)
- U. N. Shrivastava, K. suetsugu, S. Nagano, H. Fritzsche, Y. Nagao, K. Karan
Soft Matter, 16, 1190 - 1200 (2020)
DOI: 10.1039/C9SM01731C
Repository: http://hdl.handle.net/10119/17075
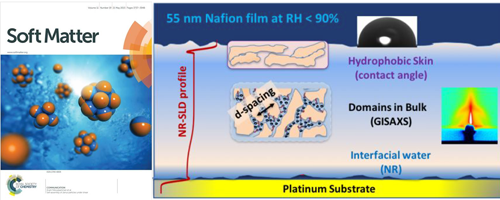
- [3]
- Studies on structural and ionic transport in biopolymer electrolytes based alginate-LiBr
- A. F. Fuzlin, Y. Nagao, I. I. Misnon, A. S. Samsudin
Ionics, 26, 1923 - 1938 (2020)
DOI: 10.1007/s11581-019-03386-7
- [2]
- Ionic transport studies of solid bio-polymer electrolytes based on carboxymethyl cellulose doped with ammonium acetate and its potential application as an electrical double layer capacitor
- N. M. J. Rasali, M. A. Saadiah, N. K. Zainuddin, Y. Nagao, A. S. Samsudin
Express Polym. Lett., 14, 619 - 637 (2020)
DOI: 10.3144/expresspolymlett.2020.51
- [1]
- Studies on Ionics Conduction Properties of modification CMC-PVA based Polymer Blend Electrolytes via Impedance Approach
- N. F. Mazuki, A. P. P. A. Majeed, Y. Nagao, A. S. Samsudin
Polym. Test., 81, 106234 (1-13) (2020)
DOI: 10.1016/j.polymertesting.2019.106234

2019
- [13]
- Vapochromic Luminescent Proton Conductors: Switchable Vapochromism and Proton Conduction of Luminescent Pt(II) Complexes with Proton-exchangeable Sites
- A. Kobayashi, S. Imada, Y. Shigeta, Y. Nagao, M. Yoshida, M. Kato
J. Mater. Chem. C, 7, 14923 - 14931 (2019)
DOI: 10.1039/C9TC04944D
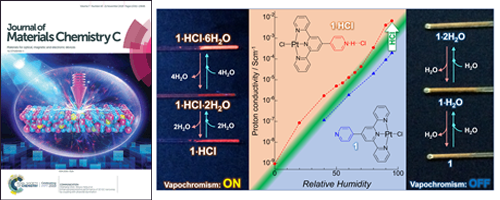
- [12]
- Potential anticancer activity of protocatechuic acid loaded in montmorillonite/Fe3O4 nanocomposites stabilized by seaweed Kappaphycus alvarezii
- Y. P. Yew, K. Shameli, S. E. B. Mohamad, Y. Nagao, S Teow, K. X. Lee, E. D. M. Isa
Int. J. Pharm., 572, 118743 (2019)
DOI: 10.1016/j.ijpharm.2019.118743
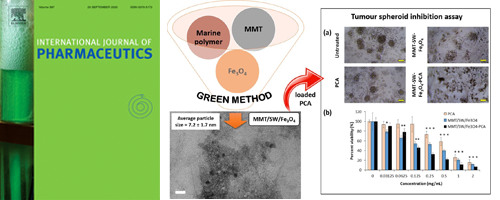
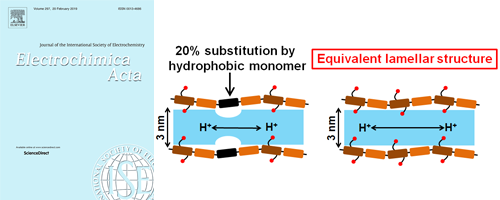
- [11]
- Introducing planar hydrophobic groups into an alkyl-sulfonated rigid polyimide and how this affects morphology and proton conductivity
- Y. Nagao*, T. Tanaka, Y. Ono, K. Suetsugu, M. Hara, G. Wang, S. Nagano, T. Abe
Electrochim. Acta, 300, 333 - 340 (2019)
DOI: 10.1016/j.electacta.2019.01.118
Relative mobility and number of density of proton carriers were discussed in sulfonated polyimide thin films.
- [10]
- Lyotropic ordering for high proton conductivity in sulfonated semialiphatic polyimide thin films (Selected as Cover Picture)
- K. Takakura, Y. Ono, K. Suetsugu, M. Hara, S. Nagano, T. Abe, Y. Nagao*
Polym. J., 51, 31 - 39 (2019)
DOI: 10.1038/s41428-018-0111-1
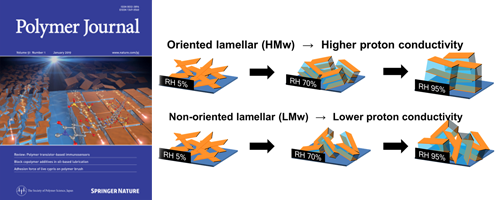
Influence of the semi-aliphatic backbone on the molecular ordering and proton conductivity was investigated compared to the rigid aromatic backbone in highly proton-conductive organized polyimide thin films. The higher molecular weight ASSPI exhibited the oriented lamellar structure in spite of lower planarity of the main chain. The proton conductivity of the oriented lamellar thin film displayed a more than half order of magnitude higher value of 1.5 × 10-1 S cm-1 than that of the non-oriented lamellar thin film (3.0 × 10-2 S cm-1) at 25°C and 95% RH. These results indicate that, in sulfonated polyimide thin films, the lamellar orientation greatly contributes to the high proton conductivity in the ASSPI thin films.
- [9]
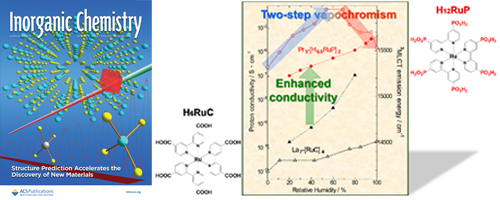
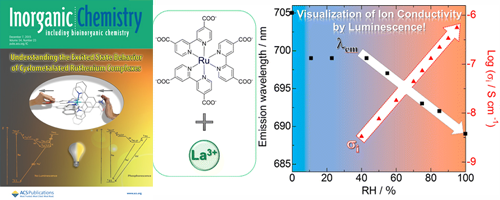
- Two-step vapochromic luminescence of proton-conductive coordination polymers composed of Ru(II)-metalloligands and lanthanide cations
- A. Kobayashi, K. Shimizu, A. Watanabe, Y. Nagao, N. Yoshimura, M. Yoshida, M. Kato
Inorg. Chem., 58, 2413 - 2421 (2019)
DOI: 10.1021/acs.inorgchem.8b02928
- [8]
- Synthesis of Mesoporous Nanoparticles via Microwave-assisted Method for Photocatalytic Degradation of Phenol Derivatives
- N. F. Jaafar, N. A. Marfur, N. W. C. Jusoh, Y. Nagao, N. H. N. Kamarudin, R. Jusoh, M. A. M. Iqbal
Malaysian J. Anal. Sci., 23, 462 - 471 (2019)
DOI: 10.17576/mjas-2019-2303-10
- [7]
- Molecularly Conductive Behavior of Blended Polymer Electrolyte-based CMC/PVA
- N. S. M. Ali, D. Zhang, Y. Nagao, A. S. Samsudin
Makara J. Technol., 23, 27 - 31 (2019)
DOI: 10.7454/mst.v23i1.3639
- [6]
- Enhancement on amorphous phase in solid biopolymer electrolytes based alginate doped NH4NO3
- N. M. J. Rasali, Y. Nagao, A. S. Samsudin
Ionics, 25, 641 - 654 (2019)
DOI: 10.1007/s11581-018-2667-3
- [5]
- Reducing crystallinity on thin film based CMC/PVA hybrid polymer for application as a host in polymer electrolytes
- M. A. Saadiah, D. Zhang, Y. Nagao, S. K. Muzakir, A. S. Samsudin
J. Non-Cryst. Solids, 511, 201 - 211 (2019)
DOI: 10.1016/j.jnoncrysol.2018.11.032
- [4]
- Electrical Properties of A Novel Solid Biopolymer Electrolyte based on Algi-nate Incorporated with Citric Acid
- A. F. A. Fuzlin , N. S. Ismail, Y. Nagao, A. S. Samsudin
Makara J. Technol., 23, 48 - 52 (2019)
DOI: 10.7454/mst.v23i1.3643
- [3]
- X-ray diffraction and spectroscopic studies of microwave synthesized mesoporous titania nanoparticles for photodegradation of 2-chlorophenol under visible light
- N. F. Jaafar, Z. H. Ahmad, N. W. C. Jusoh, Y. Nagao
AIP Conf. Proc., 2068, 010081 (2019)
DOI: 10.1063/1.5089380
- [2]
- Effect of Molecular Orientation to Proton Conductivity in Sulfonated Polyimides with bent backbones
- Y. Nagao*, K. Ohno, S. Tsuyuki, K. Suetsugu, M. Hara, S. Nagano
Mol. Cryst. Liq. Cryst., 686, 84 - 91 (2019)
DOI: 10.1080/15421406.2019.1648041
- [1]
- Anisotropic proton conductivity of poly(aspartic acid) thin films
- Y. Nagao*, J. Matsui
Mater. Today Proc., 17, 953 - 958 (2019)
DOI: 10.1016/j.matpr.2019.06.448
2018
- [7]
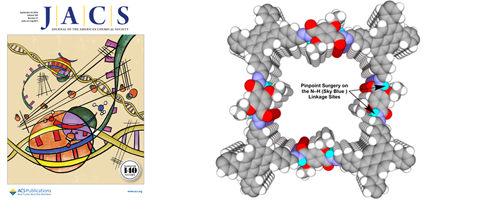
- Light-Emitting Covalent Organic Frameworks: Fluorescence Improving via Pinpoint Surgery and Selective Switch-On Sensing of Anions
- Z. Li, N. Huang, L. H. Lee, Y. Feng, S. Tao, Q. Jiang, Y. Nagao, S. Irle, D. Jiang
J. Am. Chem. Soc., 140, 12374 - 12377 (2018)
DOI: 10.1021/jacs.8b08380
- [6]
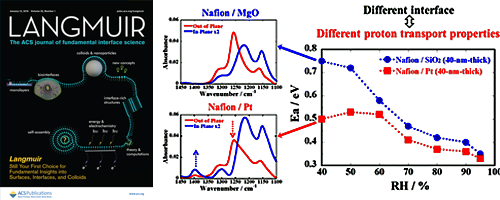
- Proton Conductivity and Oriented Structure of Nafion Thin Films on the Au-deposited Surface and MgO Substrate
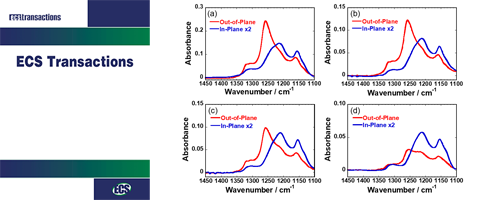
- Y. Nagao*, A. Enta, A. Suwansoontorn, Y. Ono
ECS Trans., 88, 249 - 258 (2018)
DOI: 10.1149/08801.0249ecst
In-plane proton conductivity and interfacial structure of Nafion thin films on the Au-deposited surface and MgO(100) substrate were investigated.
- [5]
- Immittance Response on Carboxymethyl Cellulose Blend with Polyvinyl Alcohol-Doped Ammonium Bromide-Based Biopolymer Electrolyte
- N. Mazuki, Y. Nagao, A. S. Samsudin
Makara J. Technol., 22, 167 - 170 (2018)
DOI: 10.7454/mst.v22i3.3638
- [4]
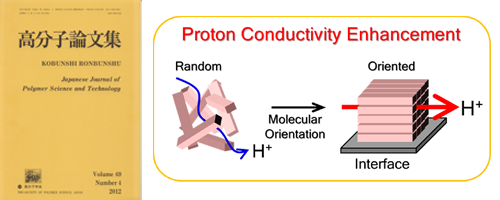
- 分子配向と組織構造を利用した高プロトン伝導性高分子薄膜の研究 (Review)
- Y. Nagao*
高分子論文集 (Japanese Journal of Polymer Science and Technology), 75, 576 - 587 (2018)
DOI: 10.1295/koron.2018-0012
高プロトン伝導性高分子には,エネルギー変換,センサー,触媒,アクチュエータなどさまざまな用途がある.含水により高プロトン伝導性を示す高分子の分子設計は,強酸性基を骨格に導入し,含水により親疎水の相分離構造を形成させ,親水チャネルを使ってプロトンを輸送させることに基づいている.Nafionのような高プロトン伝導性高分子は,相分離構造を示すものの,長距離秩序をもたないために,構造とプロトン伝導性の相関の議論は容易ではなかった.筆者らの研究グループは基板界面を利用して高分子を配向させ,分子配向がプロトン伝導性に与える影響を調べてきた.本報では,Nafionの薄膜化によるプロトン伝導度の低下,アミドオリゴマー薄膜のプロトン伝導度の基板依存性,ポリペプチド薄膜の分子配向によるプロトン伝導度の向上,およびスルホン化ポリイミド薄膜が有するリオトロピック液晶性による組織構造と高プロトン伝導性の相関を述べる.
- [3]
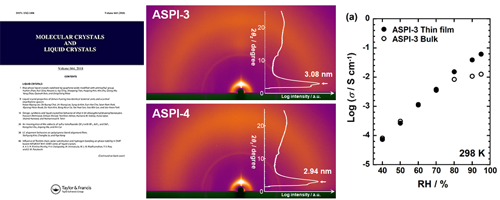
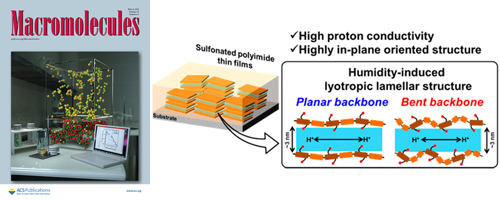
- High proton conduction of organized sulfonated polyimide thin films with planar and bent backbones
- Y. Ono, R. Goto, M. Hara, S. Nagano, T. Abe, Y. Nagao*
Macromolecules, 51, 3351 - 3359 (2018)
DOI: 10.1021/acs.macromol.8b00301
Repository: http://hdl.handle.net/10119/15728
Alkyl sulfonated polyimides (ASPIs) with bent main chain structure were newly synthesized to investigate relations between the higher order structure and proton transport properties. Proton conductivity of all polyimide thin films was greater than 10–2 S/cm. Grazing-incidence small-angle X-ray scattering (GI-SAXS) revealed that both planar and bent ASPI thin films exhibited humidity-induced lyotropic lamellar structure. Results demonstrate that sulfonated alkyl side chains contribute strongly to the lyotropic LC property, which enhances molecular orderings and proton conductivity by water uptake.
- [2]
- Core-Shell Cylinder (CSC) Nanotemplates Comprised of Mussel-Inspired Catechol-Containing Triblock Copolymers for Silver Nanoparticle Arrays and Ion Conductive Channels
- H. Yabu, S. Nagano, Y. Nagao
RSC Advances, 8, 10627 - 10632 (2018)
DOI: 10.1039/c8ra00630j
This is the first report about the synthesis of triblock copolymers containing catechol groups by reversible-addition fragmentation transfer (RAFT) polymerization. The synthesized triblock copolymer forms a core–shell cylinder (CSC) phase-separated structure, in which PVCa domains located the surface of cylinders, and it works as a template for silver nanoparticle arrays and a proton conductive channel.
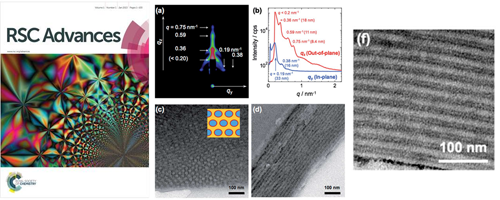
- [1]
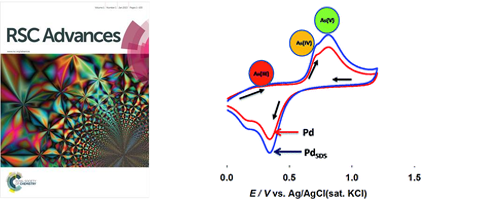
- Electrochemical Oxidation of As(III) on Pd immobilized Pt surface: kinetics and sensing performance
- Md. M. Alam, Md. A. Rashed, Md. M. Rahman, M. M. Rahman, Y. Nagao, M. A. Hasnat
RSC Advances, 8, 8071 - 8079 (2018)
DOI: 10.1039/c7ra12576c
Pd nanoparticles were electrochemically immobilized on Pt surface in presence of sodium dodecyl sulfate (SDS) molecules to study electrokinetics of arsenite oxidation reactions and corresponding sensing activities. The experimental results revealed the As(III) oxidation proceeds using a consecutive pathway; As(III) → As(IV) → As(V).
2017
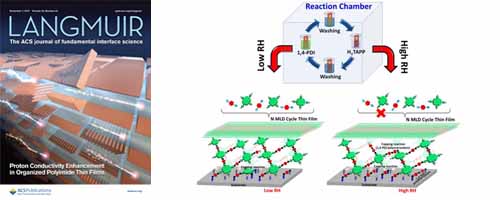
- [6]
- Multilayer Growth of Porphyrin-Based Polyurea Thin Film Using Solution-Based Molecular Layer Deposition Technique
- S. M. Nizam Uddin, Y. Nagao*
Langmuir, 33, 12777 - 12784 (2017)
DOI: 10.1021/acs.langmuir.7b03450
Repository: http://hdl.handle.net/10119/15727
We demonstrated a solution-based molecular layer deposition (MLD) approach to prepare porphyrin-based covalent organic molecular networks on a modified substrate surface using the urea coupling reaction at room temperature.
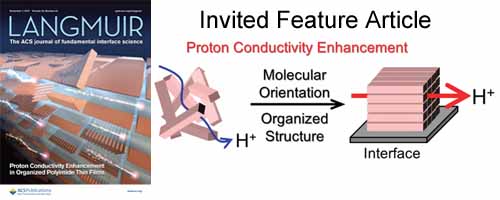
- [5]
- Proton-Conductivity Enhancement in Polymer Thin Films (Invited Feature Article, Selected as Cover Picture)
- Y. Nagao*
Langmuir, 33, 12547 - 12558 (2017)
DOI: 10.1021/acs.langmuir.7b01484
Repository: http://hdl.handle.net/10119/15729
This Feature Article presents a new approach to enhancing the proton conductivity of the polymer thin films using an interface that can modify the degrees of freedom for a polymer structure through interaction between the substrate surface and polymers.
- [4]
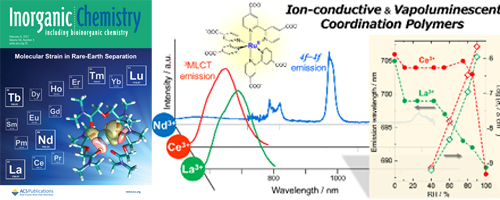
- Development of Ion-Conductive and Vapoluminescent Porous Coordination Polymers Composed of Ruthenium(II) Metalloligand
- A. Watanabe, A. Kobayashi, E. Saitoh, Y. Nagao, S. Omagari, T. Nakanishi, Y. Hasegawa, W. M. C. Sameera, M. Yoshida, M. Kato
Inorg. Chem., 56, 3005 - 3013 (2017)
DOI: 10.1021/acs.inorgchem.6b03123
- [3]
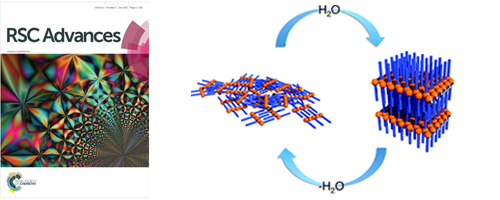
- In-plane oriented highly ordered lamellar structure formation of poly(N-dodecylacrylamide) induced by humid annealing
- Y. Hashimoto, T. Sato, R. Goto, Y. Nagao, M. Mitsuishi, S. Nagano, J. Matsui
RSC Advances, 7, 6631 - 6635 (2017)
DOI: 10.1039/C6RA27994E
- [2]
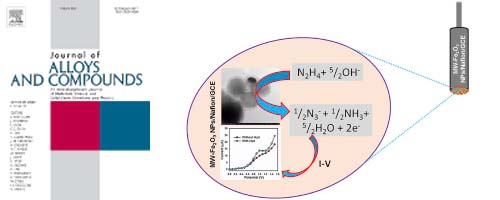
- Fabrication of hydrazine sensor based on silica-coated Fe2O3 magnetic nanoparticles prepared by a rapid microwave irradiation method
- H. Akhter, J. Murshed, M. A. Rashed, Y. Oshima, Y. Nagao, M. M. Rahman, A. M. Asiri, M. A. Hasnat, M. N. Uddin, I. A. Siddiquey
J. Alloy. Compd., 698, 921 - 929 (2017)
DOI: 10.1016/j.jallcom.2016.12.266
A facile, efficient and rapid method for fabrication of silica-coated Fe2O3 magnetic nanoparticles (NPs) by a microwave (MW) irradiation method is reported.
- [1]
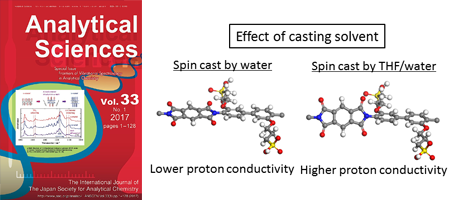
- Effect of Casting Solvent on Interfacial Molecular Structure and Proton Transport Characteristics of Sulfonated Polyimide Thin Films (Selected as HOT article)
- Y. Nagao*, K. Krishnan, R. Goto, M. Hara, S. Nagano
Anal. Sci. (Special Issue), 33, 35 - 39 (2017)
DOI: 10.2116/analsci.33.35
Crystalline and amorpous parts are discussed in the sulfonated polyimide thin films to understand the proton transport property.
2016
- [6]
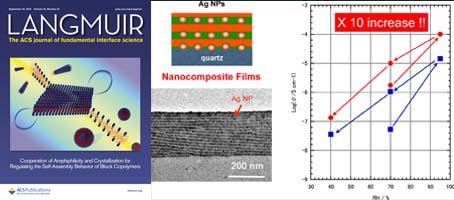
- Proton Conductivities of Lamellae-forming Bio-inspired Block Copolymer Thin Films Containing Silver Nanoparticles
- H. Yabu, J. Matsui, M. Hara, S. Nagano, Y. Matsuo, Y. Nagao
Langmuir, 32, 9484 - 9491 (2016)
DOI: 10.1021/acs.langmuir.6b02521
Size-controlled metal nanoparticles (NPs) were spontaneously
formed. The proton
conductivity of well-aligned lamellae structured PVCa-b-PSt films with
Ag NPs was evaluated. We found that the proton conductivity of PVCab-PSt
film was increased 10-fold by the addition of Ag NPs into the
proton conduction channels filled with catechol moieties.
- [5]
- Metal-Organic Coordination Network Thin Film by Surface-Induced Assembly
- S. Laokroekkiat, M. Hara, S. Nagano, Y. Nagao*
Langmuir, 32, 6648 - 6655 (2016)
DOI: 10.1021/acs.langmuir.6b01251
Repository: http://hdl.handle.net/10119/14269
The growth of metal–organic coordination network thin films on surfaces has been pursued extensively and intensively to manipulate the molecular arrangement.
- [4]
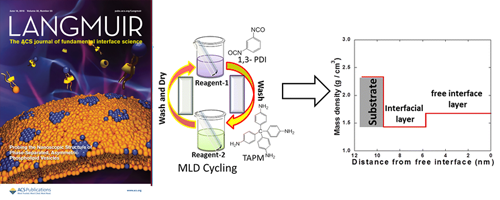
- Fabrication and Characterization of Cross-linked Organic Thin Films with Nonlinear Mass Densities
- M. A. Rashed, S. Laokroekkiat, M. Hara, S. Nagano, Y. Nagao*
Langmuir, 32 , 5917 - 5924 (2016)
DOI: 10.1021/acs.langmuir.6b00540
Repository: http://hdl.handle.net/10119/14268
The preparation of urea (bonded) cross-linked multilayer thin films by sequential deposition of bifunctional and tetrafunctional molecular building blocks is demonstrated. Molecular ordering and mass density increase in the surface normal direction of the substrate with a certain number of deposited layers.
- [3]
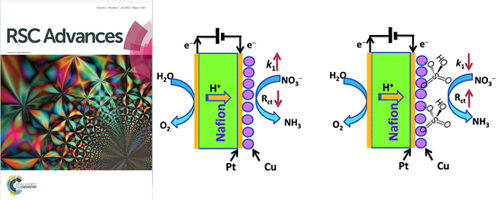
- Inverse effects of supporting electrolytes on the electrocatalytic nitrate reduction activities in a Pt|Nafion|Pt-Cu type reactor assembly
- M. A. Hasnat, J. A. Safwan, M. A. Rashed, Z. Rahman, M. M. Rahman, Y. Nagao, A. M. Asiri
RSC Advances, 6, 11609 - 11617 (2016)
DOI: 10.1039/C5RA22645G
The effects of Cl− and SO42− ions on the electrocatalytic nitrate reduction activities in a sandwich-type reactor assembly are illustrated. It was noticed that a Pt|Nafion|Pt–Cu assembly offers its best efficiency in the absence of any supporting electrolytes.
- [2]
- Hydrophobized Plant Polyphenol: Self-Assembly and Promising Antibacterial, Adhesive, and Anticorrosion Coatings
- D. Payra, M. Naito, Y. Fujii, Y. Nagao
Chem. Commun., 52, 312 - 315 (2016)
DOI: 10.1039/C5CC07090B
Hydrophobized plant polyphenol can be easily prepared by rational and controlled etherification of highly abundant aromatic hydroxyls with linear alkyl chains. The resultant organo-soluble polyphenols spontaneously formed fibrous structures and unravelled to be potential adhesive, anticorrosion, and antibacterial coatings.

- [1]
- Interfacial Structure and Proton Conductivity of Nafion at the Pt-deposited Surface
- Y. Ono, Y. Nagao*
Langmuir, 32, 352 - 358 (2016)
DOI: 10.1021/acs.langmuir.5b02623
Repository: http://hdl.handle.net/10119/14270
Understanding the Nafion-Pt interface structure is important because fuel cell reactions occur at the three-phase boundary. However, the interface structure of the Nafion-Pt interface remains unclear. In this study, relationship between the proton transport property and thin film structure on the Pt-deposited surface at the three-phase boundary for fuel cells is discussed. Thickness dependence of the degree of orientation for this OP band was observed at the Nafion-Pt interface.
2015
- [3]
- Visualization of Ion-conductivity: Vapochromic Luminescence of an Ion-conductive Ru(II)-metalloligand-based Porous Coordination Polymer
- A. Watanabe, A. Kobayashi, E. Saitoh, Y. Nagao, M. Yoshida, M. Kato
Inorg. Chem., 54(23), 11058 - 11060 (2015)
DOI: 10.1021/acs.inorgchem.5b02077
An vapochromic luminescence enables visualization of the ion conductivity of the material by the color of the luminescence.
- [2]
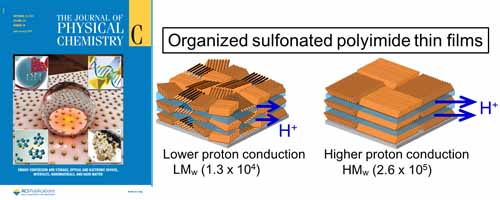
- Influence of Molecular Weight on Molecular Ordering and Proton Transport in Organized Sulfonated Polyimide Thin Films
- K. Krishnan, H. Iwatsuki, M. Hara, S. Nagano, Y. Nagao*
J. Phys. Chem. C, 119(38), 21767 - 21774 (2015)
DOI: 10.1021/acs.jpcc.5b03292
Repository: http://hdl.handle.net/10119/14271
The effect of molecular ordering and ordered domain size on molecular weight dependent proton conductivity in well-organized sulfonated polyimide (SPI) thin films has been investigated.
- [1]
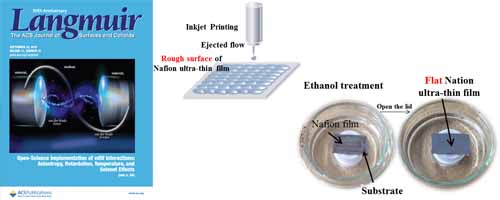
- Modification for Uniform Surface of Nafion Ultra-thin Film Deposited by Inkjet Printing
- Y. Guo, Y. Ono, Y. Nagao*
Langmuir, 31 (37), 10137 - 10144 (2015)
DOI: 10.1021/acs.langmuir.5b02395
Repository: https://dspace.jaist.ac.jp/dspace/handle/10119/13804?locale=en
We demonstrated the utilization of practical film-forming technique inkjet printing to fabricate a Nafion ultra-thin film less than 10nm thickness. Post-treatment in an ethanol vapor atmosphere exhibited a significant effect on flattening and homogenizing the film surface morphology. Results show that the well-distributed Nafion ultra-thin film modified by ethanol vapor annealing manifested much-improved proton conductivity.
2014
- [5]
- Anomalous Enhancement of Proton Conductivity for Water Molecular Clusters Stabilized in Interstitial Spaces of Porous Molecular Crystals
- M. Tadokoro, Y. Ohhata, Y. Shimazaki, S. Ishimaru, T. Yamada, Y. Nagao, T. Sugaya, K. Isoda, Y. Suzuki, H. Kitagawa, H. Matsui
Chemistry - A European Journal, 20(42), 13698 - 13709 (2014)
DOI: 10.1002/chem.201402900
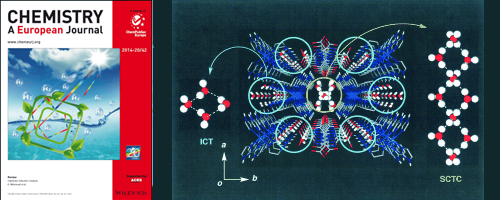
Proton conductivity of crystallized water clusters confined within low-dimensional nanoporous materials
- [4]
- Influence of Confined Polymer Structure on Proton Transport Property in Sulfonated Polyimide Thin Films
- K. Krishnan, T. Yamada, H. Iwatsuki, M. Hara, S. Nagano, K. Otsubo, O. Sakata, A. Fujiwara, H. Kitagawa, Y. Nagao*
Electrochemistry, 82(10), 865 - 869 (2014)
DOI: 10.5796/electrochemistry.82.865
The organized structure and proton transport property in sulfonated polyimide (SPI) thin film have been studied. The high proton conductivity of 2.8 × 10−1 S cm−1 at 80°C and 90% RH was achieved in the highly oriented SPI thin film. In contrast, the bulk SPI showed the drop of conductivity value at high relative humidity region, which is due to the significant structural disorder accompanied by the strong interaction between the sulfonic acid side chains and water molecules.
- [3]
- Surface proton transport of fully protonated poly(aspartic acid) thin films on quartz substrates
- Y. Nagao*, T. Kubo
Applied Surface Science, 323, 19 - 24 (2014)
DOI: 10.1016/j.apsusc.2014.06.085
Repository: https://dspace.jaist.ac.jp/dspace/handle/10119/12335?locale=en
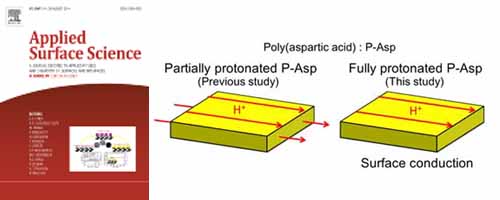
Proton transport of fully protonated poly(aspartic acid) thin film was investigated.
The thin film structure differed greatly from the partially protonated one.
Proton transport occurs on the surface, not inside of the thin film.
This result contributes to biological transport systems such as bacteriorhodopsin.
- [2]
- Proton conductivity enhancement in oriented, sulfonated polyimide thin films
- K. Krishnan, H. Iwatsuki, M. Hara, S. Nagano, Y. Nagao*
J. Mater. Chem. A, 2(19), 6895 - 6903 (2014)
DOI: 10.1039/c4ta00579a
Repository: https://dspace.jaist.ac.jp/dspace/handle/10119/12610?locale=en
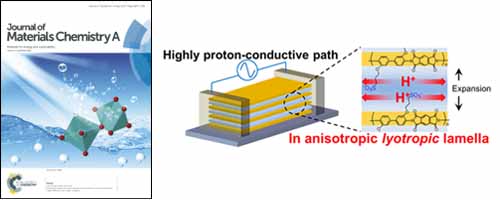
Studies of proton transport in confined thin polymer electrolytes are essential for providing additional information regarding the structure–property relationships of such materials. Sulfonated polyimide film confined to a thickness of approximately 530 nm shows significant proton conductivity enhancement to a value of 2.6 × 10−1 S/cm (95% RH at 298 K).
- [1]
- Effects of Nafion impregnation using inkjet printing for membrane electrode assemblies in polymer electrolyte membrane fuel cells
- Z. Wang, Y. Nagao*
Electrochim. Acta, 129, 343 - 347 (2014)
DOI: 10.1016/j.electacta.2014.02.133
Repository: https://dspace.jaist.ac.jp/dspace/handle/10119/12148?locale=en
We present a method of using inkjet printing to deposit Nafion ionomer as the transport media onto catalyst layer made into membrane electrode assemblies (MEAs) for polymer electrolyte fuel cells (PEMFCs).
2013
- [3]
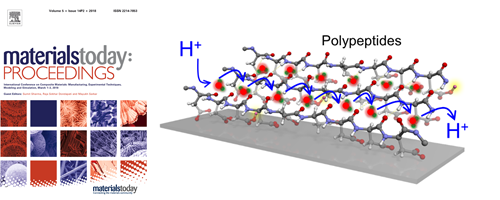
- Enhancement of Proton Transport in an Oriented Polypeptide Thin Film
- Y. Nagao*, J. Matsui, T. Abe, H. Hiramatsu, H. Yamamoto, T. Miyashita, N. Sata, H. Yugami
Langmuir, 29, 6798 - 6804 (2013)
DOI: 10.1021/la400412f
Repository: https://dspace.jaist.ac.jp/dspace/handle/10119/12061?locale=en
Proton transport properties of a partially protonated poly(aspartic acid)/sodium polyaspartate (P-Asp) were investigated. A remarkable enhancement of proton conductivity has been achieved in the thin film. Proton conductivity of 60-nm-thick thin film prepared on MgO(100) substrate was 3.4 × 10–3 S cm–1 at 298 K.
- [2]
- Substrate dependence of the proton transport and oriented structure in oligo[(1,2propanediamine)-alt-(oxalic acid)] thin films (Editor's choice)
- Y. Nagao*
Chem. Lett., 42, 468 - 470 (2013)
DOI: 10.1246/cl.130019
Repository: https://dspace.jaist.ac.jp/dspace/handle/10119/11564?locale=en
The molecular orientation in the thin film could be correlated with the proton-transport properties.
- [1]
- Highly Oriented Sulfonic Acid Groups in a Nafion Thin Film on Si Substrate
- Y. Nagao*
J. Phys. Chem. C, 117, 3294 - 3297 (2013)
DOI: 10.1021/jp311622p
Repository: https://dspace.jaist.ac.jp/dspace/handle/10119/12062?locale=en
Nafion thin film on Si substrate had a highly oriented structure with the sulfonic acid groups at the side chain. The lower proton conductivity of the Nafion thin film was related with these highly oriented structures.
2012
- [3]
- Surface electronic structure of BaZr1-xYxO3-δ by soft-X-ray spectroscopy
- H. Tohru, F. Iguchi, Y. Nagao, N. Sata, Y. S. Liu, P. A. Glans, J. Guo, H. Yugami
Transactions of the Materials Research Society of Japan, 37, 575 - 578 (2012)
- [2]
- A study on the plasma-treated surfaces of MgO(100) and quartz substrates by infrared multiple-angle incidence resolution spectrometry
- Y. Nagao*
e-Journal of Surface Science and Nanotechnology, 10, 229 - 233 (2012)
DOI: 10.1380/ejssnt.2012.229
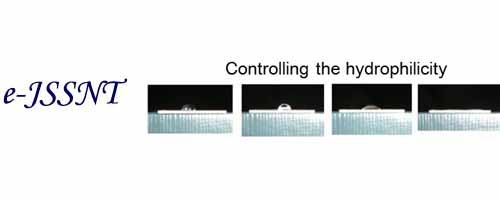
Contact angle measurements and an infrared p-polarized multiple-angle incidence resolution spectrometry (p-MAIRS) technique were performed on MgO(100) and quartz substrates.
- [1]
- Proton transport property of Nafion thin films on MgO(100) with anisotropic molecular structure
- Y. Nagao*
e-Journal of Surface Science and Nanotechnology, 10, 114 - 116 (2012)
DOI: 10.1380/ejssnt.2012.114
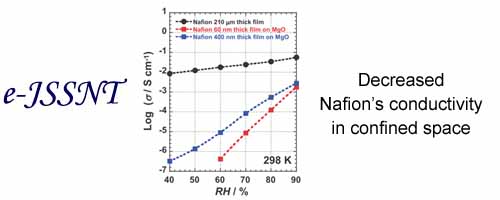
The proton conductivity of two thin films were quite lower values compared to that of the commercial Nafion membrane, and the thickness dependence of the proton conductivity was also observed.
2011
- [4]
- Photocurable Electrolyte Based on Sulfonated Poly(ether ether ketone)
- Y. Nagao*, T. Iwadera, N. Sata, F. Iguchi, H. Yugami
Solid State Ionics, 204-205, 35 - 40 (2011)
DOI: 10.1016/j.ssi.2011.09.013
Repository: https://dspace.jaist.ac.jp/dspace/handle/10119/10628?locale=en
Protonconcutive photocurable electrolytes were prepared. Patterning of micro-structure on the photocured membrane was also demonstrated by using the property of the photocurable electrolytes.
- [3]
- Catalytic activity of carbon-supported iridium oxide for oxygen reduction reaction as a Pt-free catalyst in polymer electrolyte fuel cell
- C. H. Chang, T. S. Yuen, Y. Nagao*, H. Yugami
Solid State Ionics, 197, 49 - 51 (2011)
DOI: 10.1016/j.ssi.2011.06.015
Repository: https://dspace.jaist.ac.jp/dspace/handle/10119/10627?locale=en
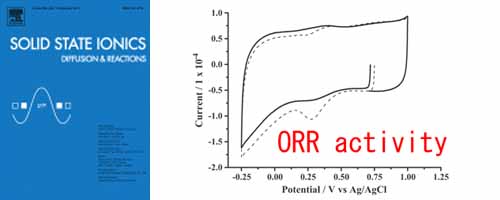
Iridium oxide supported on Vulcan XC-72 carbon black (IrO2/C) as a cathode catalyst for polymer electrolyte fuel cell (PEFC) has been characterized by transmission electron microscopy (TEM), X-ray diffraction (XRD), cyclic voltammetry (CV). It was found that IrO2/C had high oxygen reduction reaction (ORR) activity. IrO2/C catalyst would be potential candidates for use as cathode catalyst in PEFC.
- [2]
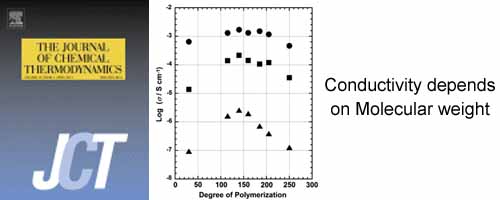
- Proton transport properties of poly(aspartic acid) with different average molecular weight
- Y. Nagao*, Y. Imai, J. Matsui, T. Ogawa, T. Miyashita
The Journal of Chemical Thermodynamics, 43, 613 - 616 (2011)
DOI: 10.1016/j.jct.2010.11.016
Repository: https://dspace.jaist.ac.jp/dspace/handle/10119/10629?locale=en
We synthesized seven partially protonated poly(aspartic acids)/sodium polyaspartates (P-Asp) with different average molecular weights to study their proton transport properties.
- [1]
- Proton concentration in 15mol% Y-doped BaZrO3 proton conductors prepared at various temperatures
- F. Iguchi, Y. Nagao, N. Sata, H. Yugami
Solid State Ionics, 192, 97 - 100 (2011)
DOI: 10.1016/j.ssi.2010.05.046

2010
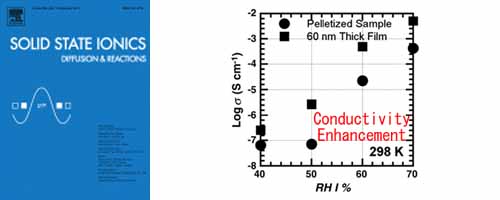
- [3]
- Synthesis and Proton transport property of Poly(aspartic acid) thin film on SiO2 substrate
- Y. Nagao*, F. Iguchi, N. Sata, H. Yugami
Solid State Ionics, 181, 206 - 209 (2010)
DOI: 10.1016/j.ssi.2009.03.011
Repository: https://dspace.jaist.ac.jp/dspace/handle/10119/10630?locale=en
Poly(aspartic acid) and its thin film were synthesized to study proton transport properties. The proton conductivity of the thin film is one order of magnitude higher than that of the pelletized Poly(aspartic acid). The activation energies for the proton conduction of the pelletized Poly(aspartic acid) and thin film are 0.65 and 0.53 eV, respectively.
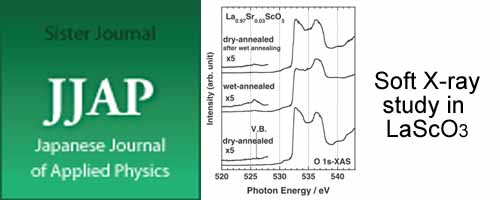
- [2]
- Electronic Structure of Sr-doped LaScO3 Single Crystal Annealed in Different Atmospheres
- Y. Nagao*, J. Liu, F. Iguchi, T. Higuchi, N. Sata, H. Yugami
Japanese Journal of Applied Physics, 49, 010208 (2010)
DOI: 10.1143/JJAP.49.010208
The electronic structure near the top of the valence band and the bottom of the conduction band for Sr-doped LaScO3 single crystal was investigated by X-ray absorption spectroscopy (XAS).
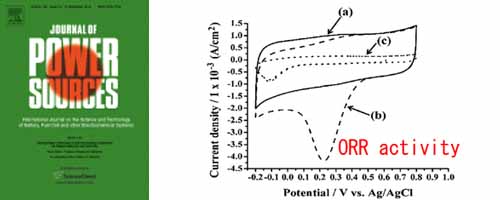
- [1]
- Electrocatalytic activity of Iridium Oxide nanoparticles coated on carbon for oxygen reduction as cathode catalyst in PEFC
- C. H. Chang, T. S. Yuen, Y. Nagao*, H. Yugami
Journal of Power Sources, 195, 5938 - 5941 (2010)
DOI: 10.1016/j.jpowsour.2010.01.049
The iridium oxide nanoparticles supported on Vulcan XC-72 porous carbon were prepared for cathode catalyst in polymer electrolyte fuel cell (PEFC). The catalyst has been characterized by transmission electron microscopy (TEM) and in PEFC tests. The iridium oxide nanoparticles, which were uniformly dispersed on carbon surface, were 2–3 nm in diameter. With respect to the oxygen reduction reaction (ORR) activity was also studied by cyclic voltammetry (CV), revealing an onset potential of about 0.6 V vs. an Ag/AgCl electrode. The ORR catalytic activity of this catalyst was also tested in a hydrogen–oxygen single PEFC and a power density of 20 mW cm−2 has been achieved at the current density of 68.5 mA cm−2. This study concludes that carbon-supported iridium oxide nanoparticles have potential to be used as cathode catalyst in PEFC.
2009
- [4]
- Proton conductivity of oligomeric poly[(1, 2-propane-diamine)-alt-(oxalic acid)] thin films on Al2O3
- Y. Nagao*, N. Naito, F. Iguchi, N. Sata, H. Yugami
e-Journal of Surface Science and Nanotechnology, 7, 530 - 532 (2009)
DOI: 10.1380/ejssnt.2009.530
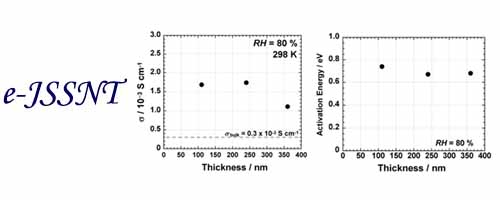
Proton transport properties of three thin films (110, 240, and 360 nm thick films) on R-plane (1102) sapphire substrates for oligomeric poly[(1, 2-propanediamine)-alt-(oxalic acid)] were investigated. The maximum proton conductivity of the thin film is 1.7 × 10-3 S cm-1 at the RH of 80%, which is five times higher than that of the bulk sample (3.0 × 10-4 S cm-1). The activation energies of the 110, 240, and 360 nm thick films are found to 0.74, 0.67, and 0.68 eV at the RH of 80%, respectively.
- [3]
- Synthesis of oligomeric poly[(1, 2-propanediamine)-alt-(oxalic acid)] and anomalous proton conductivities of the thin films
- Y. Nagao*, N. Naito, F. Iguchi, N. Sata, H. Yugami
Solid State Ionics, 180, 589 - 591 (2009)
DOI: 10.1016/j.ssi.2008.09.022
Repository: https://dspace.jaist.ac.jp/dspace/handle/10119/10631?locale=en
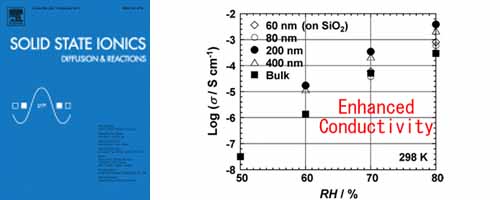
New proton-conductive polyamide oligomers, oligomeric poly[(1, 2-propanediamine)-alt-(oxalic acid)], were synthesized to investigate the proton transport properties of bulk and thin films. The proton conductivity of thin film is relatively higher than that of bulk sample. Thickness dependence of the proton conductivity was observed in these thin films.
- [2]
- Synthesis and Proton Transport Property of Poly(aspartic acid) Thin Film on MgO(100) Substrate
- Y. Nagao*, M. Ando, H. Maekawa, C. H. Chang, F. Iguchi, N. Sata,
ECS Transactions, 16, 401 - 406 (2009)
DOI: 10.1149/1.3242254
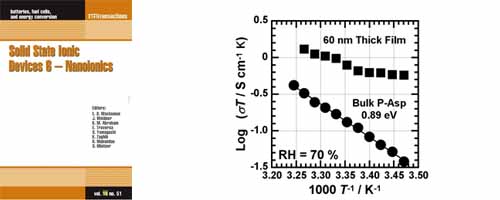
- [1]
- The relationship between chemical composition distributions and specific grain boundary conductivity in Y-doped BaZrO3 proton conductors
- F. Iguchi, T. Tsurui, N. Sata, Y. Nagao, H. Yugami
Solid State Ionics, 180, 563 - 568 (2009)
DOI: 10.1016/j.ssi.2008.12.006

2008
- [3]
- Electronic Structure of La1-xSrxScO3 Probed by Soft-X-Ray Absorption Spectroscopy
- T. Higuchi, Y. Nagao, J. Liu, F. Iguchi, N. Sata, T. Hattori, H. Yugami
Journal of Applied Physics, 104, 076110 (2008)
DOI: 10.1063/1.2999641
- [2]
- Synthesis and protonic conductivity of the oligomeric amides with different average molecular weights
- Y. Nagao*, A. Haneda, N. Naito, F. Iguchi, N. Sata, H. Yugami
Solid State Ionics, 179, 1142 - 1145 (2008)
DOI: 10.1016/j.ssi.2008.01.051
- [1]
- Proton conductivity of biopolymer-platinum nanoparticle composite under high humidity
- M. Fujishima, H. Takatori, K. Yamai, Y. Nagao, H. Kitagawa, K. Uchida
Journal of Materials Science, 43, 9, 3130 - 3134 (2008)
DOI: 10.1007/s10853-008-2509-1
2007
- [1]
- Performance of BaZrO3 based Proton Conductors as an Electrolyte for Intermediate Temperature Operating SOFC
- F. Iguchi, T. Tokikawa, T. Miyoshi, T. Tsurui, Y. Nagao, N. Sata, H. Yugami
ECS Transactions, 7, 2331 - 2336 (2007)
DOI: 10.1149/1.2729352
2006
- [1]
- Structures and phase transition of multi-layered water nanotube confined to nanochannels
- M. Tadokoro, S. Fukui, T. Kitajima, Y. Nagao, S. Ishimaru, H. Kitagawa, K. Isobe, K. Nakasuji
Chemical Communications, 12, 1274 - 1276 (2006)
DOI: 10.1039/B516807D
2005
- [1]
- Preparation and proton transport property of N, N'-diethyldithiooxamidatocopper coordination polymer
- Y. Nagao, T. Kubo, K. Nakasuji, R. Ikeda, T. Kojima, H. Kitagawa
Synthetic Metals, 154, 89 - 92 (2005)
DOI: 10.1016/j.synthmet.2005.07.006
A novel proton-conductive copper coordination polymer, (H5C2)2dtoaCu (R2dtoaH2 = dithiooxamide derivatives), was synthesized. The proton conductivity (σp) was 4.2 × 10−6 S cm−1 under the RH of 100% and the ionic transport number was more than 0.99. The σp of (HOH4C2)2dtoaCu was two orders of magnitude higher than that of (H5C2)2dtoaCu, which would be derived from the existence of-OH groups in the alkyl substituent R.
2003
- [4]
- A new proton-conductive copper coordination polymer, (HOC3H6)2dtoaCu (dtoa = dithiooxamide)
- Y. Nagao, R. Ikeda, K. Iijima, T. Kubo, K. Nakasuji, H. Kitagawa
Synthetic Metals, 135-136, 283 - 284 (2003)
DOI: 10.1016/S0379-6779(02)00914-1
A new copper coordination polymer, (HOC3H6)(2)dtoaCu (dtoa = dithiooxamide), was synthesized. The a.c. conductivity measurements with an impedance analyzer were carried out for (HOC3H6)(2)dtoaCu in order to estimate the proton conductivity (sigmap).
- [3]
- Substituent effect on the magnetic properties of copper coordination polymers with dithiooxamide and N, N'-bis-(hydroxyethyl)dithiooxamide
- M. Fujishima, Y. Nagao, R. Ikeda, S. Kanda, H. Kitagawa
Synthetic Metals, 133-134, 433 - 435 (2003)
DOI: 10.1016/S0379-6779(02)00281-3
- [2]
- Highly proton-conductive copper coordination polymers
- Y. Nagao, M. Fujishima, R. Ikeda, S. Kanda, H. Kitagawa
Synthetic Metals, 133-134, 431 - 432 (2003)
DOI: 10.1016/S0379-6779(02)00282-5
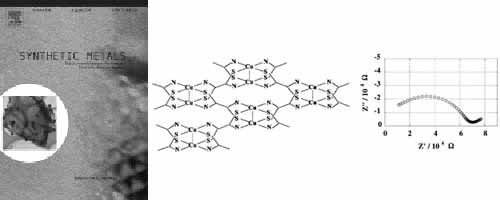
AC conductivity measurements were carried out for a two-dimensional coordination polymer, N,N′-bis-(2-hydroxyethyl)dithiooxamidatocopper(II) with an impedance analyzer in order to estimate the protonic conductivity. This polymer was found to exhibit a high protonic conduction of σp=1.2×10−5 S cm−1 under relative humidity of 83% and 300 K.
- [1]
- Highly proton-conductive copper coordination polymer, H2dtoaCu (H2dtoa = dithiooxamide anion)
- H. Kitagawa, Y. Nagao, M. Fujishima, R. Ikeda, S. Kanda
Inorganic Chemistry Communications, 6, 346 - 348 (2003)
DOI: 10.1016/S1387-7003(02)00749-9
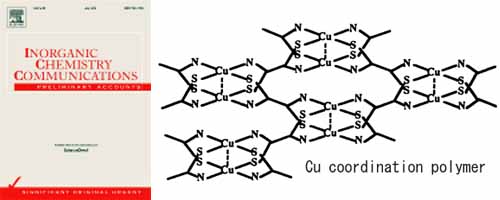
Room-temperature proton conductivity, coordination geometry, and pore-diameter distribution of the title Cu(II) coordination polymer have been investigated by AC conductivity, EXAFS, and N2 adsorption isotherm measurements. The AC proton conductivity (σp) obtained from Cole–Cole plot analysis under relative humidity of 75% and 300 K exhibits a considerable high value of as a room-temperature σp. The mechanism of proton conduction seems to be similar to that of a proton-exchange membrane, Nafion, which contains much cluster of water molecules in the porous space of the polymer. The present Cu coordination polymer was revealed to possess porous space of about 6 Å, which includes much water molecules more than 10H2O per unit cell. The dimeric Cu(II) square-planar coordination geometry was confirmed by EXAFS analysis using synchrotron radiation source at SPring-8.
2002
- [1]
- Complex-Plane Impedance Study on A Hydrogen-Doped Copper Coordination Polymer,N,N'-bis-(2-hydroxyethyl)dithiooxamidatocopper(II)
- Y. Nagao, R. Ikeda, S. Kanda, Y. Kubozono, H. Kitagawa
Molecular Crystals and Liquid Crystals, 79, 89 - 94 (2002)
DOI: 10.1080/713738672
AC conductivity measurements with an impedance analyzer were carried out for a hydrogen-doped coordination polymer, N , N '-bis-(2-hydroxyethyl)dithiooxamidatocopper(II), in order to estimate the protonic condctivity. The log proton conductiivty was linearly increased from 2.6x10-9 to 2.2 x 10-6 S cmwith relative humidity (RH) from 45 to 100% at 300 K. A slight hysteresis of protonic conductivity was observed upon increasing and decreasing RH, which implies that H3O+ is generated by a reaction between water molecule and acid-base polymer near RH∼100%.