Novel Liquid Metal Nanoparticles for Cancer Photoimmunotherapy
Researchers develop a novel cancer theranostic agent that can covert light energy to heat and in turn destroy cancer cells
Liquid metals (LM) have unique physicochemical properties, making them suitable for varied applications in different domains. Researchers from Japan have developed a novel water-dispersible light-activatable LM nanostimulant that can activate T and dendritic cells. It is highly immunogenic and has robust photothermal activity to specifically destroy cancer cells, with minimal cytotoxicity. Additionally, it is fluorescently tagged to function as a reporter to identify and eliminate tumors in vivo for cancer diagnosis.
Liquid metals (LM) such as pure gallium (Ga) and Ga-based alloys are a new class of materials with unique physicochemical properties. One of the most prominent applications of LMs is photothermal therapy against cancer, in which functional LM nanoparticles convert light energy to heat energy, thus killing cancerous cells. LM-based phototherapy is superior to traditional cancer therapy owing to its high specificity, repeatability, and low side effects.
In a new cutting-edge study, Associate Professor Eijiro Miyako and his colleagues from Japan Advanced Institute of Science and Technology (JAIST) synthesized multifunctional Ga-based nanoparticles that combine cancer phototherapy with immunotherapy. The synthesized novel LM nanoparticle (PEG-IMIQ-LM) contains a eutectic gallium-indium (EGaIn) LM alloy and an immunological modulator imiquimod (IMIQ), both embedded inside a biocompatible surfactant DSPE-PEG2000-NH2. The detailed findings of their study were published in Advanced Functional Materials.
"We believe that the convergence of nano-immuno engineering and LM technology could provide a promising modality to trigger ideal immune responses for advancing cancer immunotherapy. In this study, we report light-activatable multifunctional LM nanoparticles with immunostimulants to combine photothermal therapy with immunotherapy," says Dr. Miyako, while discussing the team's motivation to conduct this study.
First, the research team prepared water-dispersible LM nanoparticles through a simple one-step sonication process using DSPE-PEG2000-NH2 to introduce IMIQ. This is considered a huge breakthrough, as EGaIn LM is inherently a water-immiscible material. Further investigations confirmed that LM disintegrates to ensure IMIQ delivery to the target. Moreover, the prepared nanoparticle displayed a linear increase in absorbance in the near-infrared (NIR) region at 808 nm, confirming its optically activatable nature.
When the aqueous solution of the LM nanoparticle was irradiated by the NIR laser (808 nm), the team observed a notable increase in the temperature of the solution, which was proportional to the increase in the nanoparticle concentration. These findings confirmed that PEG-IMIQ-LM nanoparticle was a robust and stable photothermal drug carrier, suitable for immunotherapy.
Further experiments revealed that LM nanoparticles were extremely safe and did not cause cytotoxicity in human fibroblast (MRC5) and mouse colon cancer (Colon26) cells. To assess the degree of internalization and distribution of the particles, a fluorescent dye known as indocyanine green (ICG) was introduced into the particle through sonication resulting in PEG-ICG-IMIQ-LM particle. Fluorescent (FL) microscopy equipped with a laser beam demonstrated that the LM particle displayed strong fluorescence at various NIR wavelengths and immediately destroyed the Colon26 cells. Thus, LM particles could not only efficiently deliver the immunomodulant, but could also enable their real time tracking and eliminate specific cancer cells.
Finally, the team developed a multifaceted LM immune nanostimulator for cancer theranostics. To do so, they added anti-programmed death ligand-1 antibody (Anti-PD-L1), one of the most promising immune checkpoint inhibitors, to the existing fluorescent LM nanoparticle. The modified particle, Anti-PD-L1‒PEG-ICG-IMIQ-LM, was dispersed efficiently with significant fluorescence. With increasing time post irradiation, the tumor surface temperature increased linearly, indicative of the antitumor effect of the nanoparticle.
Addition of Anti-PD-L1 onto the nanoparticle enabled binding of the LM particle to PD-L1 on the cancer cells, marking them for phagocytosis by macrophages and dendritic cells (DC). Laser-induced Anti-PD-L1-PEG-IMIQ-LM particles exhibited the highest and complete cancer removal, along with faster healing and recovery.
Moreover, when the tumor recurred, mice treated with laser-induced Anti-PD-L1-PEG-IMIQ-LM particles displayed sustained antitumor effectiveness and prolonged survival.
While discussing the future implications of the study, Dr. Miyako muses, "We believe that these synergistic immunological effects and optical nanofunctions of LMs have wide therapeutic applications and might contribute to innovative cancer theranostic technologies. We are hopeful that this technology will be available for clinical trials in 10 years."
We cannot wait to see how the team's findings contribute to novel and effective treatments against cancer.
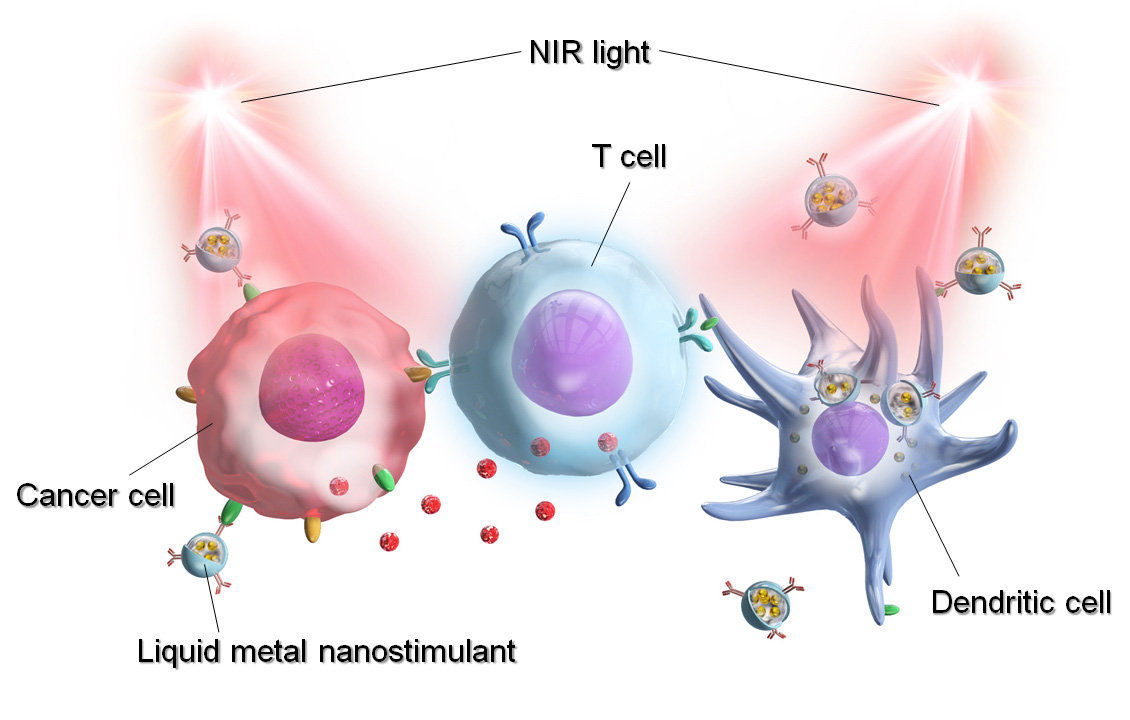
Title: Light-activatable liquid metal nanostimulant in cancer therapy
Caption: A versatile liquid metal (LM) gallium-indium alloy has been used to develop a novel LM nanoparticle that harbors an immunomodulant and an immune checkpoint inhibitor, Anti-PD-L1. Upon irradiation by near infra-red light, Anti-PD-L1 specifically binds to the cancer cell, while immunostimulants activate T and dendritic cells. This synergistic activation coupled with the photothermal effect effectively eliminates the cancer cell almost immediately.
Image Credit: Eijiro Miyako from JAIST
License Type: Original Content
Reference
| Title of original paper: | Light-Activatable Liquid Metal Immunostimulants for Cancer Nanotheranostics |
| Authors: | Yun Qi, Mikako Miyahara, Seigo Iwata, Eijiro Miyako* |
| Journal: | Advanced Functional Materials |
| DOI: | 10.1002/adfm.202305886 |
Funding information
This work was financially supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Scientific Research (A) (Grant number 23H00551), JSPS KAKENHI Grant-in-Aid for Challenging Research (Pioneering) (Grant number 22K18440), the Japan Science and Technology Agency for Adaptable and Seamless Technology Transfer Program through Target-driven R&D (Grant Number JPMJTR22U1), Institute for Fermentation, Osaka (IFO), and the Uehara Memorial Foundation.
August 4, 2023
