Towards Targeted Catalyst Optimization: Identifying Rate-Limiting Steps in Photocatalysis
A temperature- and light intensity-based methodology yields the Onset Intensity for Temperature Dependence --- a newly proposed parameter that sheds light on the rate-limiting step in photocatalysis
Photocatalysis is limited by either the charge supply or the charge transfer process. Identifying the correct rate-limiting step is crucial for optimizing reactions. Researchers from JAIST have developed a novel diagnostic method that uses variations in reaction temperature and light intensity to pinpoint the Onset Intensity for Temperature Dependence, a key bottleneck metric. This approach effectively distinguishes between charge supply- and charge transfer-limited regimes.
Photocatalysis--- a chemical reaction driven by light in the presence of a photocatalyst--- is poised to play a key role in next-generation technologies, including hydrogen production through water splitting, carbon dioxide reduction, and environmental purification by utilizing sunlight. Thanks to these promising applications, photocatalysis is attracting attention as a major tool for building sustainable cities and societies.
However, photocatalysis involves a series of interconnected processes--- light absorption, carrier excitation and transport, and surface redox reactions--- that proceed in a continuous manner. This makes it challenging to identify the rate-limiting step of the overall reaction. As a result, optimizing photocatalytic reactions for maximum efficiency remains a significant challenge.
In a recent breakthrough, researchers from the Graduate School of Advanced Science and Technology at the Japan Advanced Institute of Science and Technology, Japan, led by Research Assistant Professor Yohei Cho and Professor Toshiaki Taniike, have introduced a novel methodology to pinpoint the bottleneck metrics and thus determine rate-limited regimes in photocatalysis. Their findings have been published in the Journal of Materials Chemistry A.
"In this study, we categorized photocatalytic reactions into two key processes: charge supply, which refers to the supply of excited carriers to the surface, and charge transfer, which involves redox (oxidation-reduction) reactions. Since surface reactions are more sensitive to temperature changes, we introduced the Onset Intensity for Temperature Dependence, or OITD, as a crucial metric. It marks the point at which the reaction rate begins to respond to temperature, allowing us to clearly distinguish which of the two processes is rate-limiting," explains Dr. Cho.
The researchers measured photocatalytic reaction rates under varying temperatures and light intensities to identify the OITD and determine whether the reaction was limited by charge supply and charge transfer. Using the decomposition of methylene blue as a model reaction, they studied titanium dioxide (TiO2) and zinc oxide (ZnO) as representative photocatalysts. TiO2 exhibited temperature dependence only at high light intensity, suggesting that the material is relatively more constrained by charge supply. In contrast, ZnO showed temperature sensitivity even at lower light intensity, suggesting that its performance is relatively more limited by surface reactions. These findings reveal distinct rate-limiting behaviors for different materials. Furthermore, the study highlighted that enhancing surface accessibility through nanoparticle formation plays a more important role in improving charge supply than increasing crystallinity. This insight offers a concrete design principle for optimizing photocatalytic material design.
Dr. Cho highlights the broader impact of their findings: "Our diagnostic method supports the rational design of photocatalysts for solar-driven hydrogen production, carbon dioxide reduction, and environmental remediation. It enables rapid screening of materials and informs targeted optimization strategies--- such as co-catalyst loading or nanostructuring--- for efficient and practical solar energy utilization technologies. Ultimately, this can accelerate the development of sustainable energy and environmental technologies, potentially contributing to carbon neutrality and cleaner water and air."
In conclusion, OITD offers a straightforward yet powerful diagnostic for identifying whether a photocatalytic reaction is limited by charge supply or surface charge transfer, paving the way for smarter catalyst design and improved reaction efficiency.
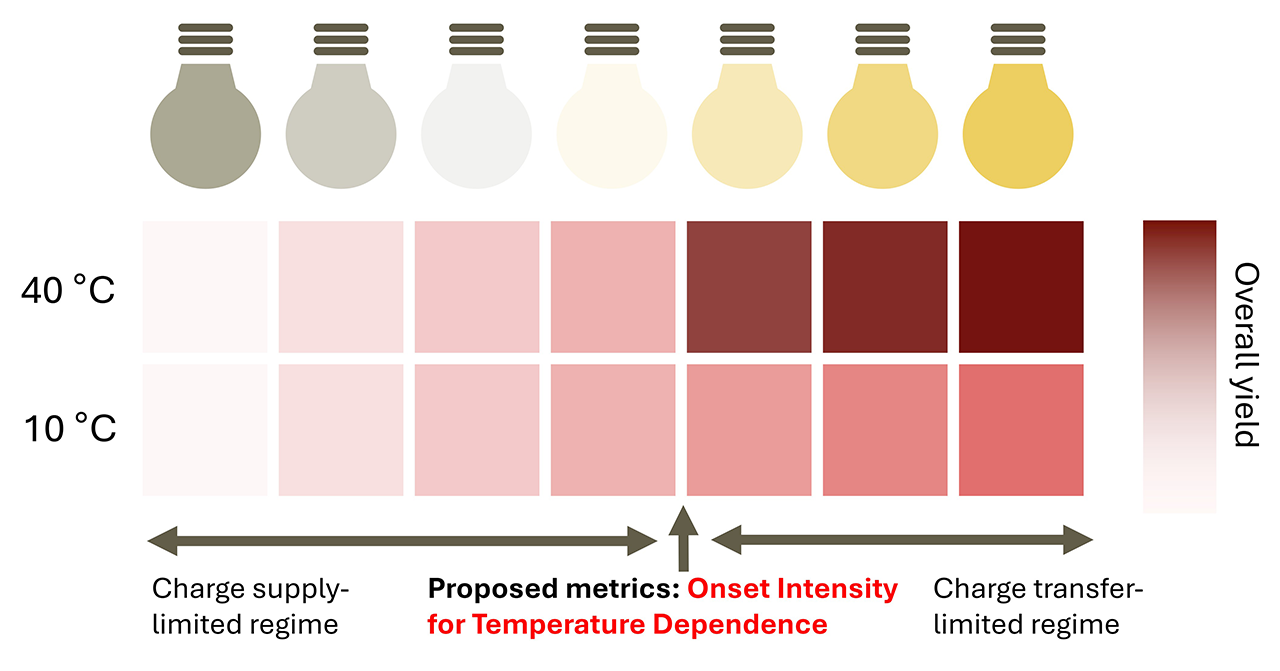
Image title: Pinpointing the Photocatalytic Bottleneck with Onset Intensity for Temperature Dependence
Image caption: By analyzing the reaction rates of photocatalysts like titanium dioxide and zinc oxide under varying temperatures and light intensities, researchers distinguish whether the rate-limiting step is charge supply or surface charge transfer--- providing a powerful tool for targeted photocatalyst design.
Image credit: Yohei Cho from JAIST.
Image license: Original Content
Usage restrictions: Cannot be reused without permission.
Reference
| Title of original paper: | Identifying Rate-Limiting Steps in Photocatalysis: A Temperature- and Light Intensity-Dependent Diagnostic of Charge Supply vs. Charge Transfer |
| Authors: | Yohei Cho*, Kyo Yanagiyama, Poulami Mukherjee, Panitha Phulkerd, Krishnamoorthy Sathiyan, Emi Sawade, Toru Wada, and Toshiaki Taniike* |
| Journal: | Journal of Materials Chemistry A |
| DOI: | 10.1039/D5TA00415B |
Funding information
This research was supported by the Grant-in-Aid for Scientific Research (No. 24KJ1201), the JST Next Generation Researcher Challenging Research Program (No. JPMJSP2102), and the Leave a Nest Research Grant KYOCERA Corporation Award.
May 12, 2025
